Difference between revisions of "Chromatography"
(→About Chromatography) |
(→About Chromatography) |
||
| Line 43: | Line 43: | ||
: [[Chromatography]] relies on different [[chemical]]s experiencing different [[force]]s of [[attraction]] between the [[solvent]] used and the [[chromatography]] paper. The [[solvent]] is referred to as the '[[Mobile Phase|mobile phase]]' and acts to carry the [[chemical]] along as the [[solvent]] moves through the [[chromatography]] paper (mobile because it makes the chemical move). The [[chromatography]] paper is referred to as the '[[Stationary Phase|stationary phase]]' and acts to hold the [[chemical]]s in place (stationary because it stops them from moving). | : [[Chromatography]] relies on different [[chemical]]s experiencing different [[force]]s of [[attraction]] between the [[solvent]] used and the [[chromatography]] paper. The [[solvent]] is referred to as the '[[Mobile Phase|mobile phase]]' and acts to carry the [[chemical]] along as the [[solvent]] moves through the [[chromatography]] paper (mobile because it makes the chemical move). The [[chromatography]] paper is referred to as the '[[Stationary Phase|stationary phase]]' and acts to hold the [[chemical]]s in place (stationary because it stops them from moving). | ||
: Each [[chemical]] has a [[Retention Factor|Retention Factor (R<sub>f</sub> number)]] which is a [[ratio]] of how far the [[chemical]] moves along the paper compared to how far the [[solvent]] moves along the paper. The larger this [[Retention Factor]] the greater the [[force]] of [[attraction]] experienced by the [[chemical]] to the paper. The [[chemical]] is 'retained' in place. | : Each [[chemical]] has a [[Retention Factor|Retention Factor (R<sub>f</sub> number)]] which is a [[ratio]] of how far the [[chemical]] moves along the paper compared to how far the [[solvent]] moves along the paper. The larger this [[Retention Factor]] the greater the [[force]] of [[attraction]] experienced by the [[chemical]] to the paper. The [[chemical]] is 'retained' in place. | ||
| − | : | + | : [[Retention Factor|R<sub>f</sub>]] numbers are unique to each [[chemical]] and can be used to [[Separating Mixtures|separate]] and identify the [[chemical]]. |
| + | |||
| + | ===Method=== | ||
| + | ====Identifying Chemicals by Comparison==== | ||
| + | ====Identifying Chemicals by R<sub>f</sub> Number==== | ||
Revision as of 12:17, 22 January 2019
Contents
Key Stage 3
Meaning
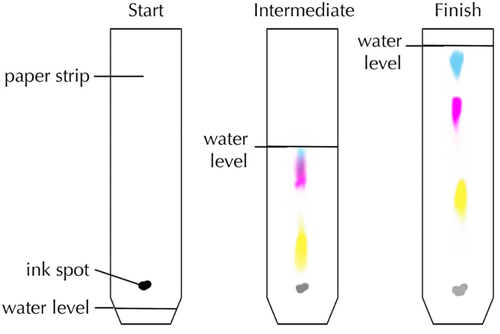
Chromatography is a method used to separate and identify different solutes found in a solution.
About Chromatography
- When more than one solute is dissolved in a solvent chromatography can be used to separate them.
- Chromatography experiments are often done with colourful solutes which can be seen easily.
| This diagram shows how black ink is made of three different inks in solution. |
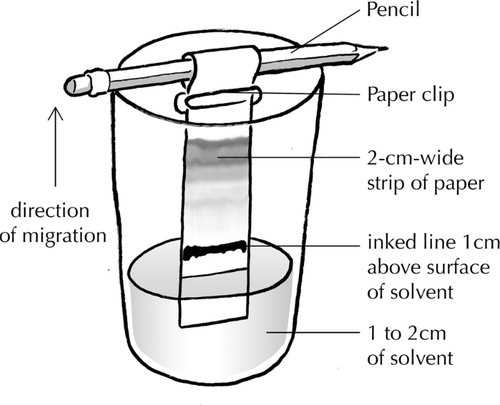
Method
|
Key Stage 4
Meaning
Chromatography is a technique used to separate and identify different solutes found in a solution.
About Chromatography
Chromatography can be used for:
- Separating and identifying two or more solutes from a solution.
- Identifying pure from impure substances.
Chromatography cannot be used for:
- Separating separating liquids in solution - Fractional Distillation
- Separating an insoluble solid from a soluble solid - Filtration
- Separating solutes from a solvent in solution. - Evaporation (Crystallisation) or Distillation.
- Chromatography relies on different chemicals experiencing different forces of attraction between the solvent used and the chromatography paper. The solvent is referred to as the 'mobile phase' and acts to carry the chemical along as the solvent moves through the chromatography paper (mobile because it makes the chemical move). The chromatography paper is referred to as the 'stationary phase' and acts to hold the chemicals in place (stationary because it stops them from moving).
- Each chemical has a Retention Factor (Rf number) which is a ratio of how far the chemical moves along the paper compared to how far the solvent moves along the paper. The larger this Retention Factor the greater the force of attraction experienced by the chemical to the paper. The chemical is 'retained' in place.
- Rf numbers are unique to each chemical and can be used to separate and identify the chemical.