Contents
Key Stage 3
Meaning
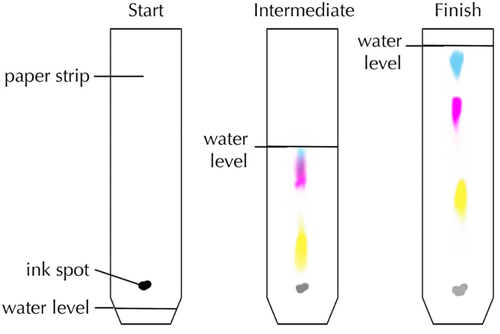
Chromatography is a method used to separate and identify different solutes found in a solution.
About Chromatography
- When more than one solute is dissolved in a solvent chromatography can be used to separate them.
- Chromatography experiments are often done with colourful solutes which can be seen easily.
| This diagram shows how black ink is made of three different inks in solution. |
Method
|
Key Stage 4
Meaning
Chromatography is a technique used to separate and identify different solutes found in a solution.
About Chromatography
Chromatography can be used for:
- Separating and identifying two or more solutes from a solution.
- Identifying pure from impure substances.
Chromatography cannot be used for:
- Separating separating liquids in solution - Fractional Distillation
- Separating an insoluble solid from a soluble solid - Filtration
- Separating solutes from a solvent in solution. - Evaporation (Crystallisation) or Distillation.
- Chromatography relies on different chemicals experiencing different forces of attraction between the solvent used and the chromatography paper. The solvent is referred to as the 'mobile phase' and acts to carry the chemical along as the solvent moves through the chromatography paper (mobile because it makes the chemical move). The chromatography paper is referred to as the 'stationary phase' and acts to hold the chemicals in place (stationary because it stops them from moving).
- Each chemical has a Retention Factor (Rf number) which is a ratio of how far the chemical moves along the paper compared to how far the solvent moves along the paper. The larger this Retention Factor the greater the force of attraction experienced by the chemical to the paper. The chemical is 'retained' in place.
- Rf numbers are unique to each chemical and can be used to separate and identify the chemical.
Method
Identifying Chemicals by Comparison
The chromatogram from an experiment can be compared against chromatograms of known chemicals to identify the chemicals in the original sample.
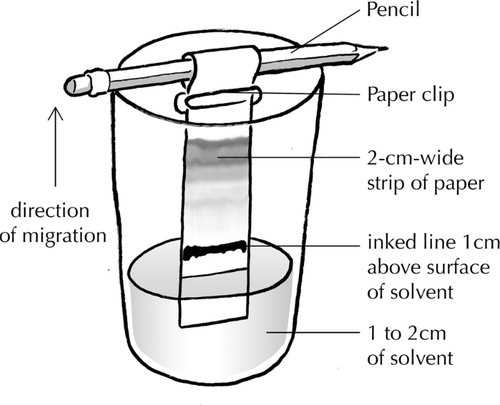
- Take a piece of chromatography paper of width 2cm and height 10cm.
- Using a ruler draw a line with pencil across the width 1cm up from the end.
- Add a dot of the unknown sample on the pencil line.
- Suspend the chromatography paper in a suitable solvent just below the pencil line with most of the paper above the solvent.
- Wait until the solvent stops rising up the paper.
- Compare this chromatogram to chromatograms of known chemicals.
Identifying Chemicals by Rf Number
The Rf values for a variety of chemicals are known. By comparing the Rf values from an experiment to the known values of different chemicals then the chemicals in the experiment may be identified.
- Take a piece of chromatography paper of width 2cm and height 10cm.
- Using a ruler draw a line with pencil across the width 1cm up from the end.
- Add a dot of the unknown sample on the pencil line.
- Suspend the chromatography paper in a suitable solvent just below the pencil line with most of the paper above the solvent.
- Wait until the solvent stops rising up the paper.
- Use a ruler to measure the distance that the solvent has traveled up the paper.
- Use a ruler to measure the distance that the chemical has traveled up the paper.
- Use the equation:
\[R_f = \frac{d_c}{d_s}\]
- Where:
- Rf = Retention Factor
- dc = distance moved by the chemical
- ds = distance moved by the solvent
- Compare this value to the values for known chemicals.