Difference between revisions of "Group 3"
| Line 4: | Line 4: | ||
===About Group 3=== | ===About Group 3=== | ||
| + | {| class="wikitable" | ||
| + | |- | ||
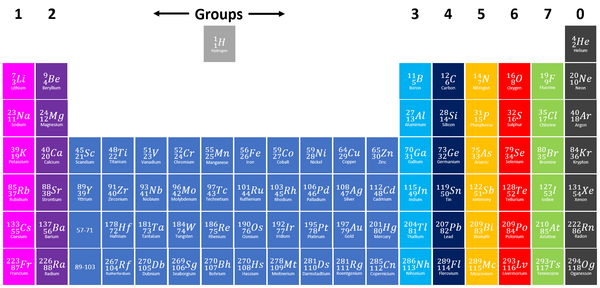
| + | |[[File:PeriodicTableGroups.png|center|600px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Group 3]] [[element]]s are shown in light blue on this [[Periodic Table]]. | ||
| + | |} | ||
: The [[element]]s in [[Group 3]] are: | : The [[element]]s in [[Group 3]] are: | ||
:*[[Boron]] | :*[[Boron]] | ||
| Line 17: | Line 23: | ||
===About Group 3=== | ===About Group 3=== | ||
: [[Group 3]] [[atom]]s can lose 3 [[electron]]s to form [[Positive Ion|positive ions]] with a [[Electrical Charge|charge]] of +3. | : [[Group 3]] [[atom]]s can lose 3 [[electron]]s to form [[Positive Ion|positive ions]] with a [[Electrical Charge|charge]] of +3. | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:PeriodicTableGroups.png|center|600px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Group 3]] [[element]]s are shown in light blue on this [[Periodic Table]]. | ||
| + | |} | ||
: The [[element]]s in [[Group 3]] are: | : The [[element]]s in [[Group 3]] are: | ||
:*[[Boron]] | :*[[Boron]] | ||
Latest revision as of 08:35, 4 April 2019
Key Stage 3
Meaning
Group 3 are elements on the Periodic Table with only 3 electrons in their outer shell.
About Group 3
| Group 3 elements are shown in light blue on this Periodic Table. |
Key Stage 4
Meaning
Group 3 are elements on the Periodic Table with only 3 electrons in their outer shell.
About Group 3
- Group 3 atoms can lose 3 electrons to form positive ions with a charge of +3.
| Group 3 elements are shown in light blue on this Periodic Table. |