Difference between revisions of "Metal"
(→About Metals) |
(→Key Stage 4) |
||
| Line 59: | Line 59: | ||
: [[Metal]]s make good [[Electrical Conductor|electrical]] and [[Thermal Conductor|thermal]] [[conductor]]s because the [[Delocalised Electron|delocalised electrons]] are free to move around the [[material]]. | : [[Metal]]s make good [[Electrical Conductor|electrical]] and [[Thermal Conductor|thermal]] [[conductor]]s because the [[Delocalised Electron|delocalised electrons]] are free to move around the [[material]]. | ||
: [[Metal]]s usually have high [[Melting Point|melting]] and [[Boiling Point|boiling]] points making them [[solid]] at [[Room Temperature|room temperature]] except for [[Mercury (Element)|mercury]] which is a [[liquid]] at [[Room Temperature|room temperature]]. | : [[Metal]]s usually have high [[Melting Point|melting]] and [[Boiling Point|boiling]] points making them [[solid]] at [[Room Temperature|room temperature]] except for [[Mercury (Element)|mercury]] which is a [[liquid]] at [[Room Temperature|room temperature]]. | ||
| + | |||
| + | ===Applications and Properties=== | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | | style="height:20px; width:100px; text-align:center;" |'''Application''' | ||
| + | | style="height:20px; width:400px; text-align:left;" |'''Properties''' | ||
| + | |- | ||
| + | | style="height:20px; width:100px; text-align:center;" |'''Application''' | ||
| + | | style="height:20px; width:400px; text-align:left;" |[[Strength (Property)|Strong]] - Does not break easily under a force. | ||
| + | |- | ||
| + | | style="height:20px; width:100px; text-align:center;" |'''Application''' | ||
| + | | style="height:20px; width:400px; text-align:left;" |[[Malleable]] - can be hammered into shape. | ||
| + | |- | ||
| + | | style="height:20px; width:100px; text-align:center;" |'''Application''' | ||
| + | | style="height:20px; width:400px; text-align:left;" |[[Ductile]] - Can be stretched into wires. | ||
| + | |- | ||
| + | | style="height:20px; width:100px; text-align:center;" |'''Application''' | ||
| + | | style="height:20px; width:400px; text-align:left;" |High [[Melting Point]] - Can be heated to high [[temperature]]s. | ||
| + | |- | ||
| + | | style="height:20px; width:100px; text-align:center;" |'''Application''' | ||
| + | | style="height:20px; width:400px; text-align:left;" |Good [[Electrical Conductor]] - [[Electricity|electricity]] can pass through it easily. | ||
| + | |- | ||
| + | | style="height:20px; width:100px; text-align:center;" |'''Application''' | ||
| + | | style="height:20px; width:400px; text-align:left;" |'''Properties''' | ||
| + | |- | ||
| + | | style="height:20px; width:100px; text-align:center;" |'''Application''' | ||
| + | | style="height:20px; width:400px; text-align:left;" |'''Properties''' | ||
| + | |} | ||
Revision as of 12:44, 27 January 2019
Contents
Key Stage 1
Meaning
Metal is a hard, smooth, shiny, bendy and opaque material.
About Metals
There are many different types of metal but they are all similar in their properties. Metals are used to make knives and forks because they are hard and smooth. Metals are used to make wires because they are bendy. Metal can be used to make hammers because it is hard and strong.
Key Stage 3
Meaning
A Metal is a a material that is a good conductor of electricity and a good conductor of thermal energy.
About Metals
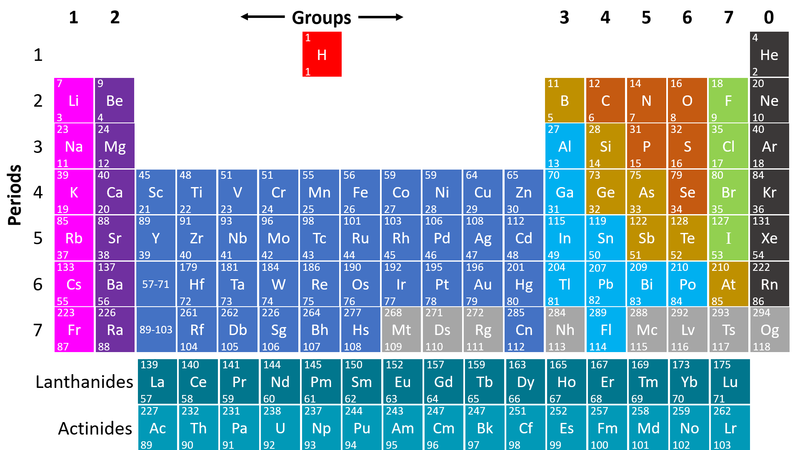
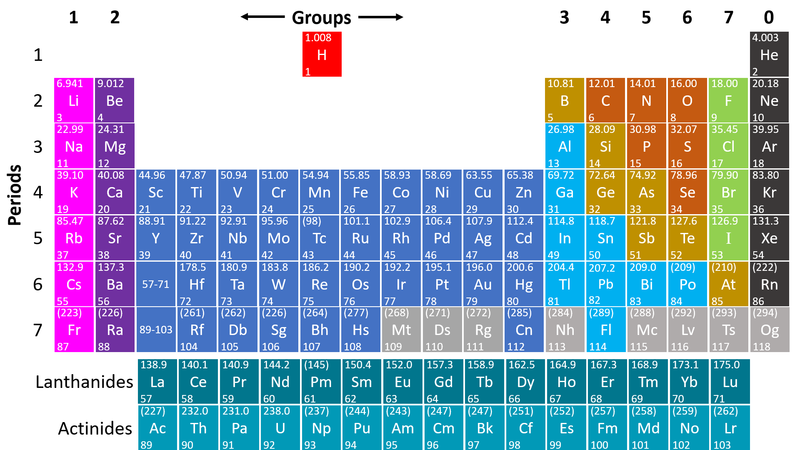
- Metals are found on the left hand side of the Periodic Table
| This Periodic Table shows the metal elements in blue and purple. |
Properties of Metals
There are several key properties of metals you should know. Metals are:
- Good conductors of electricity
- Good conductors of thermal energy
- Shiny - They have reflective surfaces when polished.
- Malleable - They can be hammered into shape.
- Ductile - They can be stretched into wires.
- Sonorous - They make a ringing sound when they are hit.
Key Stage 4
Meaning
A Metal is a a material that is a good conductor of electricity and a good conductor of thermal energy.
About Metals
- Metals are found on the left hand side of the Periodic Table
| This Periodic Table shows the metal elements in blue and purple. |
- Metals are on the left hand side of the Periodic Table because those elements lose electrons to form positive ions in compounds with non-metals.
- Metals form hydroxides when they react with water.
- Metal Oxides, Metal Carbonates and Metal Hydroxides all have pH above 7.
- Salts are metal compounds produced in Neutralisation reactions.
- Metal elements form metallic bonds in which positive ions are surrounded by a sea of delocalised electrons.
- A metal made of more than one metal element is called an alloy.
- Metals make good electrical and thermal conductors because the delocalised electrons are free to move around the material.
- Metals usually have high melting and boiling points making them solid at room temperature except for mercury which is a liquid at room temperature.
Applications and Properties
| Application | Properties |
| Application | Strong - Does not break easily under a force. |
| Application | Malleable - can be hammered into shape. |
| Application | Ductile - Can be stretched into wires. |
| Application | High Melting Point - Can be heated to high temperatures. |
| Application | Good Electrical Conductor - electricity can pass through it easily. |
| Application | Properties |
| Application | Properties |