Radioactive Decay
Key Stage 4
Meaning
Radioactive decay is when an unstable isotope emits a particle or electromagnetic wave to become more stable.
About Radioactive Decay
- During a radioactive decay an unstable isotope may emit:
- Alpha Radiation - An ionising radiation which is two protons and two neutrons (a Helium nucleus).
- Beta Radiation - An ionising radiation which is a fast moving electron ejected from the nucleus.
- Gamma Radiation - An ionising radiation which is a very high frequency electromagnetic wave emitted from the nucleus.
- Neutron Radiation - A form of indirectly ionising radiation consisting of a single neutron ejected from the nucleus. It causes ionisation by causing other elements to become unstable releasing gamma radiation.
- The rate of radioactive decay is known as the 'Half Life' which is how long it takes for half of the unstable isotopes in a sample of radioactive material to decay. This time is a constant for each type of radioactive material regardless of the quantity of unstable isotopes.
Examples
| This is a key to show the types of particles in the following decays of unstable nuclei. |
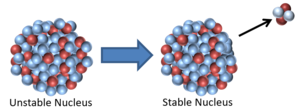
| This nucleus is unstable because it is too massive and has too few neutrons relative to protons so it decays via alpha emission reducing the atomic mass by 4 and the atomic number by 2.
\({}_Z^AX \rightarrow {}_{Z-2}^{A-4}Y + {}_2^4\alpha\) |
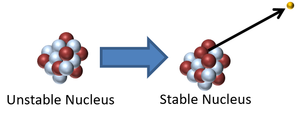
This nucleus is unstable because it is too many neutrons so it decays via beta emission in which a neutron turns into a proton increasing the atomic number by 1.
\({}_Z^AX \rightarrow {}_{Z+1}^{A}Y + {}_{-1}^0\beta\) |
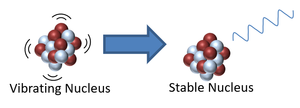
| This nucleus is unstable because it is has excess vibrational energy so it decays by emitting a gamma ray. After the decay it still has the same atomic mass and atomic number but is no longer vibrating.
\({}_Z^AX \rightarrow {}_Z^AX + {}_0^0\gamma\) |
This nucleus is unstable because it has too many neutrons relative to protons so it decays via neutron radiation reducing the atomic mass by 1.
\({}_Z^AX \rightarrow {}_{Z}^{A-1}Y + {}_0^1n\) |