Difference between revisions of "State of Matter"
(→About States of Matter) |
|||
| Line 63: | Line 63: | ||
===About States of Matter=== | ===About States of Matter=== | ||
: [[Material|Materials]] can be [[solid]], [[liquid]] or [[gas]]. | : [[Material|Materials]] can be [[solid]], [[liquid]] or [[gas]]. | ||
| − | : The '''state of matter''' can be | + | : The '''state of matter''' can be altered by changing the [[temperature]] of the [[material]] or changing the [[pressure]] on the [[material]]. |
{| class="wikitable" | {| class="wikitable" | ||
| Line 76: | Line 76: | ||
| style="height:20px; width:200px; text-align:center;" |[[Solid]]s hold their shape. | | style="height:20px; width:200px; text-align:center;" |[[Solid]]s hold their shape. | ||
|- | |- | ||
| − | |rowspan="2"|[[Particle]]s | + | |rowspan="2"|[[Particle]]s are very close together. |
| style="height:20px; width:200px; text-align:center;" |[[Solid]]s cannot be [[compressed]]. | | style="height:20px; width:200px; text-align:center;" |[[Solid]]s cannot be [[compressed]]. | ||
|- | |- | ||
| Line 83: | Line 83: | ||
| style="height:20px; width:200px; text-align:center;" |[[Particle]]s [[vibrate]]. | | style="height:20px; width:200px; text-align:center;" |[[Particle]]s [[vibrate]]. | ||
| style="height:20px; width:200px; text-align:center;" |[[Thermal Conduction]] happens best in [[solid]]s. | | style="height:20px; width:200px; text-align:center;" |[[Thermal Conduction]] happens best in [[solid]]s. | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |+Liquids | ||
| + | |- | ||
| + | |'''Particle Diagram''' | ||
| + | |'''Particle Arrangement''' | ||
| + | |'''Property''' | ||
| + | |- | ||
| + | |rowspan="4"|[[File:ParticleModelLiquid.png|center|200px]] | ||
| + | |rowspan="2"|[[Particle]]s are free to move past each other. | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Liquid]]s can be poured. | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Liquid]]s fit the shape of their container. | ||
| + | |- | ||
| + | |rowspan="2"|[[Particle]]s and close together. | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Liquid]]s cannot be [[compressed]]. | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Sound]] passes through [[liquid]]s faster than [[gas]]es. | ||
|} | |} | ||
Revision as of 18:58, 21 December 2018
Contents
Key Stage 2
Meaning
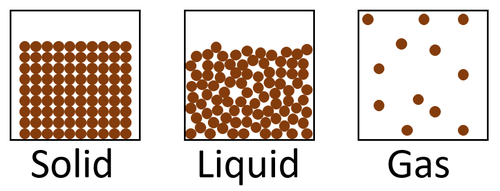
State of matter means whether a material is solid, liquid or gas.
About States of Matter
- Materials can be solid, liquid or gas.
- The state of matter can be changed by heating or cooling the material.
- Heating can turn a solid into a liquid. This is called melting.
- Heating can turn a liquid to a gas. This is called evaporating.
- Cooling can turn a gas into a liquid. This is called condensing.
- Cooling can turn a liquid into solid. This is called freezing.
| Brick is a solid material. | Water is a liquid material. | Inside the balloon is a gas called helium. |
Key Stage 3
Meaning
State of matter means whether a material is solid, liquid or gas.
About States of Matter
- Materials can be solid, liquid or gas.
- The state of matter can be changed by heating or cooling the material.
- Heating can turn a solid into a liquid by melting or it can turn a solid straight into a gas by subliming.
- Heating can turn a liquid to a gas. This is called evaporating.
- Cooling can turn a gas into a liquid by condensing or it can turn a gas into a solid by depositing.
- Cooling can turn a liquid into solid. This is called freezing.
| This diagram shows the 3 states of matter in the particle model. |
Properties of the States of Matter
| Solid | Liquid | Gas |
| Cannot be compressed. | Cannot be compressed. | Can be compressed. |
| Does not flow. | Can flow. | Can flow. |
| Holds its shape. | Fits the shape of the container. | Fits the size and shape of the container. |
Key Stage 4
Meaning
State of matter means whether a material is solid, liquid or gas.
About States of Matter
- Materials can be solid, liquid or gas.
- The state of matter can be altered by changing the temperature of the material or changing the pressure on the material.
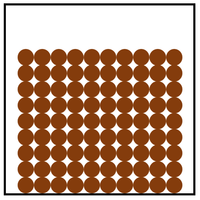
| Particle Diagram | Particle Arrangement | Property |
| Particles are in fixed positions. | Solids hold their shape. | |
| Particles are very close together. | Solids cannot be compressed. | |
| Sound passes through solids faster than liquids and gases. | ||
| Particles vibrate. | Thermal Conduction happens best in solids. |
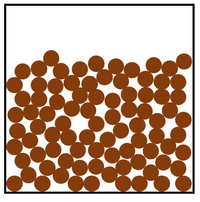
| Particle Diagram | Particle Arrangement | Property |
| Particles are free to move past each other. | Liquids can be poured. | |
| Liquids fit the shape of their container. | ||
| Particles and close together. | Liquids cannot be compressed. | |
| Sound passes through liquids faster than gases. |