Difference between revisions of "Extracting Metals by Electrolysis"
| (6 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
==Key Stage 3== | ==Key Stage 3== | ||
===Meaning=== | ===Meaning=== | ||
| − | + | '''Electrolysis''' is a way to [[Extraction of Metals|extract metals]] from [[mineral]]s when the [[metal]] is more [[Reactivity Series|reactive]] than [[Carbon]]. | |
===About Extracting Metals by Electrolysis=== | ===About Extracting Metals by Electrolysis=== | ||
| Line 9: | Line 9: | ||
|[[File:ElectrolysisCell.png|center|500px]] | |[[File:ElectrolysisCell.png|center|500px]] | ||
|- | |- | ||
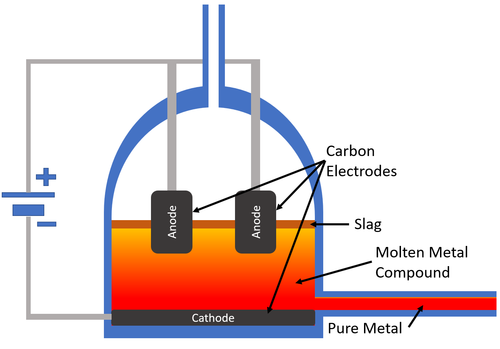
| − | | style="height:20px; width:200px; text-align:center;" |[[Carbon]] [[electrode]]s are used to pass an [[Electrical Current]] through the [[molten]] [[mineral]]. The [[metal]] collects at the [[cathode]] which carries a negative [[Electrical Charge|charge]] and the [[pure]] [[metal]] is removed from the | + | | style="height:20px; width:200px; text-align:center;" |[[Carbon]] [[electrode]]s are used to pass an [[Electrical Current]] through the [[molten]] [[mineral]]. The [[metal]] collects at the [[cathode]] which carries a negative [[Electrical Charge|charge]] and the [[pure]] [[metal]] is removed from the '''electrolysis''' cell. |
|} | |} | ||
| + | |||
| + | ==Key Stage 4== | ||
| + | ===Meaning=== | ||
| + | '''Extraction of metals by electrolysis''' is a method of obtaining a [[metal]] from a [[mineral]]. | ||
| + | |||
| + | ===About Extracting Metals by Electrolysis=== | ||
| + | : '''[[Electrolysis]]''' is usually used to [[Extraction of Metals|'''extract metals''']] when the [[metal]] is more [[Reactivity Series|reactive]] than [[Carbon]] or the [[mineral]] is [[soluble]] in [[water]] making it cheaper to '''extract by electrolysis''' than by [[smelting]]. | ||
| + | There are two possible methods used to '''extract metals by electrolysis''': | ||
| + | *Electrolysis of the Mineral in Solution - Used when the [[mineral]] is [[soluble]] in [[water]] and the [[metal]] does not [[Chemical Reaction|react]] with [[water]]. | ||
| + | *Electrolysis of the Molten Mineral - Used when the [[mineral]] is [[insoluble]] in [[water]] or the [[metal]] [[Chemical Reaction|reacts]] strongly with [[water]]. | ||
| + | : [[Aluminium]] is a special case in which the [[mineral]] is not [[dissolve]]d in [[water]] but in a [[solvent]] called Cryolite at very high [[temperature]]s. This is done because the [[mineral]] [[melt]]s at an even higher [[temperature]] than the cryolite so it is easier to [[dissolve]] it in the cryolite than to heat the [[mineral]] beyond its [[Melting Point|melting point]]. | ||
| + | |||
| + | ===Electrolysis of Molten Minerals=== | ||
| + | Used when the [[mineral]] is [[insoluble]] in [[water]] or the [[metal]] [[Chemical Reaction|reacts]] strongly with [[water]]. | ||
| + | : The [[electrolysis]] of [[molten]] [[Ionic Compound|ionic compounds]] [[Decompose (Chemistry)|decompose]]s those [[Ionic Compound|ionic compounds]] in a [[liquid]] [[State of Matter|state]]. | ||
| + | : The [[Ionic Compound|ionic compound]] must be [[heated]] and [[melting|melted]] before [[electrolysis]]. | ||
| + | : [[Non-metal]] [[ion]]ic [[element]]s or [[compound]]s will be collected at the [[anode]] where they lose their extra [[electron]]s. | ||
| + | : [[Metal]] [[ion]]s will be collected at the [[cathode]] where they gain [[electron]]s. | ||
| + | |||
| + | ===Electrolysis of Aqueous Minerals=== | ||
| + | Used when the [[mineral]] is [[soluble]] in [[water]] and the [[metal]] does not [[Chemical Reaction|react]] with [[water]]. | ||
| + | : The [[electrolysis]] of [[aqueous]] [[Ionic Compound|ionic compounds]] [[Decompose (Chemistry)|decompose]]s those [[Ionic Compound|ionic compounds]] in [[solution]]. | ||
| + | : [[Non-metal]] [[ion]]ic [[element]]s or [[compound]]s will be collected at the [[anode]] where they lose their extra [[electron]]s. | ||
| + | : [[Metal]] [[ion]]s less [[Reactivity|reactive]] than [[Hydrogen]] will be collected at the [[cathode]] where they gain [[electron]]s. | ||
Latest revision as of 15:37, 7 April 2019
Contents
Key Stage 3
Meaning
Electrolysis is a way to extract metals from minerals when the metal is more reactive than Carbon.
About Extracting Metals by Electrolysis
- Metals that are more reactive than Carbon cannot be displaced with Carbon so electrolysis is used to extract them.
| Carbon electrodes are used to pass an Electrical Current through the molten mineral. The metal collects at the cathode which carries a negative charge and the pure metal is removed from the electrolysis cell. |
Key Stage 4
Meaning
Extraction of metals by electrolysis is a method of obtaining a metal from a mineral.
About Extracting Metals by Electrolysis
- Electrolysis is usually used to extract metals when the metal is more reactive than Carbon or the mineral is soluble in water making it cheaper to extract by electrolysis than by smelting.
There are two possible methods used to extract metals by electrolysis:
- Electrolysis of the Mineral in Solution - Used when the mineral is soluble in water and the metal does not react with water.
- Electrolysis of the Molten Mineral - Used when the mineral is insoluble in water or the metal reacts strongly with water.
- Aluminium is a special case in which the mineral is not dissolved in water but in a solvent called Cryolite at very high temperatures. This is done because the mineral melts at an even higher temperature than the cryolite so it is easier to dissolve it in the cryolite than to heat the mineral beyond its melting point.
Electrolysis of Molten Minerals
Used when the mineral is insoluble in water or the metal reacts strongly with water.
- The electrolysis of molten ionic compounds decomposes those ionic compounds in a liquid state.
- The ionic compound must be heated and melted before electrolysis.
- Non-metal ionic elements or compounds will be collected at the anode where they lose their extra electrons.
- Metal ions will be collected at the cathode where they gain electrons.
Electrolysis of Aqueous Minerals
Used when the mineral is soluble in water and the metal does not react with water.
- The electrolysis of aqueous ionic compounds decomposes those ionic compounds in solution.
- Non-metal ionic elements or compounds will be collected at the anode where they lose their extra electrons.
- Metal ions less reactive than Hydrogen will be collected at the cathode where they gain electrons.