Difference between revisions of "Metal Ion"
(Created page with "==Key Stage 4== ===Meaning=== Metal Ions are positive ions found in ionic compounds and Giant Metallic Structure|giant metallic struc...") |
|||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 5: | Line 5: | ||
===About Metal Ions=== | ===About Metal Ions=== | ||
: '''Metal ions''' are formed when [[metal]] [[element]]s lose their [[electron]]s to form [[Positive Ion|positive ions]]. | : '''Metal ions''' are formed when [[metal]] [[element]]s lose their [[electron]]s to form [[Positive Ion|positive ions]]. | ||
| − | The [[charge]] on a '''metal ion''' may be determined by the [[Group]]. | + | The [[Electrical Charge|charge]] on a '''metal ion''' may be determined by the [[Group]]. |
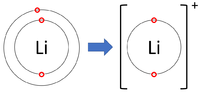
*[[Group 1]] [[Element]]s all form +1 [[ion]]s; Li<sup>+1</sup>, Na<sup>+1</sup>, K<sup>+1</sup> | *[[Group 1]] [[Element]]s all form +1 [[ion]]s; Li<sup>+1</sup>, Na<sup>+1</sup>, K<sup>+1</sup> | ||
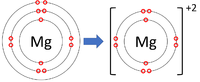
*[[Group 2]] [[Element]]s all form +2 [[ion]]s; Be<sup>+2</sup>, Mg<sup>+2</sup>, Ca<sup>+2</sup> | *[[Group 2]] [[Element]]s all form +2 [[ion]]s; Be<sup>+2</sup>, Mg<sup>+2</sup>, Ca<sup>+2</sup> | ||
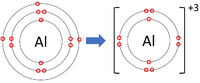
*[[Group 3]] [[Element]]s all form +3 [[ion]]s; Al<sup>+3</sup> | *[[Group 3]] [[Element]]s all form +3 [[ion]]s; Al<sup>+3</sup> | ||
| − | [[Transition Metal]] [[Element]]s can form different [[ion]]s which are shown by [[ | + | [[Transition Metal]] [[Element]]s can form different [[ion]]s which are shown by Roman Numerals; [[Iron]] can form [[Iron|Fe (II)]] which is [[Iron|Fe <sup>+2</sup>]] or [[Iron|Fe (III)]] is [[Iron|Fe<sup>+3</sup>]], |
| − | Manganese can form [[Mn | + | [[Manganese]] can form [[Manganese|Mn (II)]] which is [[Manganese|Mn<sup>+2</sup>]] or [[Manganese|Mn (IV)]] which is [[Manganese|Mn<sup>+4</sup>]]. |
: The more easily a [[metal]] [[element]] can become a '''metal ion''' the more [[reactivity|reactive]] the [[metal]]. | : The more easily a [[metal]] [[element]] can become a '''metal ion''' the more [[reactivity|reactive]] the [[metal]]. | ||
| Line 24: | Line 24: | ||
| style="height:20px; width:200px; text-align:center;" |[[Aluminium]] forms +3 [[ion]]s. | | style="height:20px; width:200px; text-align:center;" |[[Aluminium]] forms +3 [[ion]]s. | ||
|} | |} | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782945571/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945571&linkCode=as2&tag=nrjc-21&linkId=9e29fad914244909903e5e93f8a01d255 ''Metal ions, pages 89, 90, GCSE Chemistry; The Revision Guide, CGP, AQA ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Metal ions, pages 56-57, 88, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
Latest revision as of 10:31, 14 December 2019
Contents
Key Stage 4
Meaning
Metal Ions are positive ions found in ionic compounds and giant metallic structures.
About Metal Ions
- Metal ions are formed when metal elements lose their electrons to form positive ions.
The charge on a metal ion may be determined by the Group.
- Group 1 Elements all form +1 ions; Li+1, Na+1, K+1
- Group 2 Elements all form +2 ions; Be+2, Mg+2, Ca+2
- Group 3 Elements all form +3 ions; Al+3
Transition Metal Elements can form different ions which are shown by Roman Numerals; Iron can form Fe (II) which is Fe +2 or Fe (III) is Fe+3, Manganese can form Mn (II) which is Mn+2 or Mn (IV) which is Mn+4.
Examples
| Lithium forms +1 ions. | Magnesium forms +2 ions. | Aluminium forms +3 ions. |