Difference between revisions of "State of Matter"
(→About States of Matter) |
|||
| (35 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | ==Key Stage 2== | |
===Meaning=== | ===Meaning=== | ||
'''State of matter''' means whether a [[material]] is [[solid]], [[liquid]] or [[gas]]. | '''State of matter''' means whether a [[material]] is [[solid]], [[liquid]] or [[gas]]. | ||
===About States of Matter=== | ===About States of Matter=== | ||
| − | : [[Material|Materials]] can be solid, liquid or gas. | + | : [[Material|Materials]] can be [[solid]], [[liquid]] or [[gas]]. |
: The '''state of matter''' can be changed by heating or cooling the material. | : The '''state of matter''' can be changed by heating or cooling the material. | ||
| − | : Heating can turn a solid into a liquid | + | : Heating can turn a [[solid]] into a [[liquid]]. This is called [[melting]]. |
| − | : Cooling can turn a gas into a liquid | + | : Heating can turn a [[liquid]] to a [[gas]]. This is called [[evaporating]]. |
| − | + | : Cooling can turn a [[gas]] into a [[liquid]]. This is called [[condensing]]. | |
| − | + | : Cooling can turn a [[liquid]] into [[solid]]. This is called [[freezing]]. | |
{| class="wikitable" | {| class="wikitable" | ||
| − | |||
|- | |- | ||
| − | |[[File: | + | |[[File:BrickRed.png|center|200px]] |
| + | |[[File:Water.png|center|200px]] | ||
| + | |[[File:Balloon.png|center|200px]] | ||
|- | |- | ||
| − | | style="height:20px; width: | + | | style="height:20px; width:200px; text-align:center;" |[[Brick]] is a [[solid]] material. |
| − | + | | style="height:20px; width:200px; text-align:center;" |[[Water]] is a [[liquid]] material. | |
| − | + | | style="height:20px; width:200px; text-align:center;" |Inside the balloon is a [[gas]] called [[helium]]. | |
| − | |||
|} | |} | ||
| + | |||
| + | ==Key Stage 3== | ||
| + | ===Meaning=== | ||
| + | '''State of matter''' means whether a [[material]] is [[solid]], [[liquid]] or [[gas]]. | ||
| + | ===About States of Matter=== | ||
| + | : [[Material|Materials]] can be [[solid]], [[liquid]] or [[gas]]. | ||
| + | : The '''state of matter''' can be changed by heating or cooling the material. | ||
| + | : Heating can turn a [[solid]] into a [[liquid]] by [[melting]] or it can turn a [[solid]] straight into a [[gas]] by [[subliming]]. | ||
| + | : Heating can turn a [[liquid]] to a [[gas]]. This is called [[evaporating]]. | ||
| + | : Cooling can turn a [[gas]] into a [[liquid]] by [[condensing]] or it can turn a [[gas]] into a [[solid]] by [[depositing]]. | ||
| + | : Cooling can turn a [[liquid]] into [[solid]]. This is called [[freezing]]. | ||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
| − | |[[File: | + | |[[File:ParticleModelSolidLiquidGas.png|center|500px]] |
|- | |- | ||
| − | | style="height:20px; width: | + | | style="height:20px; width:200px; text-align:center;" |This [[diagram]] shows the 3 '''states of matter''' in the [[Particle Model|particle model]]. |
|} | |} | ||
| − | + | ===Properties of the States of Matter=== | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | === | ||
| − | |||
{| class="wikitable" | {| class="wikitable" | ||
| − | | | + | | style="height:20px; width:200px; text-align:center;" |'''Solid''' |
| + | | style="height:20px; width:200px; text-align:center;" |'''Liquid''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Gas''' | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |Cannot be compressed. | ||
| + | | style="height:20px; width:200px; text-align:center;" |Cannot be compressed. | ||
| + | | style="height:20px; width:200px; text-align:center;" |Can be compressed. | ||
|- | |- | ||
| − | | | + | | style="height:20px; width:200px; text-align:center;" |Does not flow. |
| + | | style="height:20px; width:200px; text-align:center;" |Can flow. | ||
| + | | style="height:20px; width:200px; text-align:center;" |Can flow. | ||
|- | |- | ||
| − | | style="height:20px; width: | + | | style="height:20px; width:200px; text-align:center;" |Holds its shape. |
| − | + | | style="height:20px; width:200px; text-align:center;" |Fits the shape of the container. | |
| − | + | | style="height:20px; width:200px; text-align:center;" |Fits the size and shape of the container. | |
| − | |||
|} | |} | ||
| + | |||
| + | ==Key Stage 4== | ||
| + | ===Meaning=== | ||
| + | '''State of matter''' means whether a [[material]] is [[solid]], [[liquid]] or [[gas]]. | ||
| + | ===About States of Matter=== | ||
| + | : [[Material|Materials]] can be [[solid]], [[liquid]] or [[gas]]. | ||
| + | : The '''state of matter''' can be altered by changing the [[temperature]] of the [[material]] or changing the [[pressure]] on the [[material]]. | ||
| + | : The [[property|properties]] of [[solid]]s, [[liquid]]s and [[gas]]es can be explained by the way the [[particle]]s inside those [[substance]]s behave. | ||
{| class="wikitable" | {| class="wikitable" | ||
| + | |+Solids | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Particle Diagram''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Particle Arrangement''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Property''' | ||
| + | |- | ||
| + | |rowspan="6"|[[File:ParticleModelSolid.png|center|200px]] | ||
| + | |rowspan="2"|[[Particle]]s are in fixed positions. | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Solid]]s hold their shape. | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Convection]] cannot happen in [[solid]]s. | ||
| + | |- | ||
| + | |rowspan="4"|[[Particle]]s are very close together. | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Solid]]s cannot be [[compressed]]. | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Sound]] passes through [[solid]]s faster than [[liquid]]s and [[gas]]es. | ||
|- | |- | ||
| − | | | + | | style="height:20px; width:200px; text-align:center;" |[[Particle]]s [[vibrate]]. |
|- | |- | ||
| − | | style="height:20px; width: | + | | style="height:20px; width:200px; text-align:center;" |[[Thermal Conduction]] happens best in [[solid]]s. |
|} | |} | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
{| class="wikitable" | {| class="wikitable" | ||
| − | |+ | + | |+Liquids |
|- | |- | ||
| − | | | + | | style="height:20px; width:200px; text-align:center;" |'''Particle Diagram''' |
| + | | style="height:20px; width:200px; text-align:center;" |'''Particle Arrangement''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Property''' | ||
|- | |- | ||
| − | | style="height:20px; width: | + | |rowspan="6"|[[File:ParticleModelLiquid.png|center|200px]] |
| − | + | |rowspan="3"|[[Particle]]s can slide past each other. | |
| − | + | | style="height:20px; width:200px; text-align:center;" |[[Liquid]]s can be poured. | |
| − | + | |- | |
| + | | style="height:20px; width:200px; text-align:center;" |[[Liquid]]s fit the shape of their container. | ||
| + | |- | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Convection]] happens in [[solid]]s. | ||
| + | |- | ||
| + | |rowspan="3"|[[Particle]]s are close together. | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Liquid]]s cannot be [[compressed]]. | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Sound]] passes through [[liquid]]s faster than [[gas]]es. | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Thermal Conduction]] happens in [[liquid]]s but not as well as in [[solid]]s. | ||
|} | |} | ||
{| class="wikitable" | {| class="wikitable" | ||
| + | |+Gases | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Particle Diagram''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Particle Arrangement''' | ||
| + | | style="height:20px; width:200px; text-align:center;" |'''Property''' | ||
| + | |- | ||
| + | |rowspan="6"|[[File:ParticleModelGas.png|center|200px]] | ||
| + | |rowspan="3"|[[Particle]]s are free to move in all directions. | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Gas]]es fit the size of their container. | ||
|- | |- | ||
| − | | | + | | style="height:20px; width:200px; text-align:center;" |[[Gas]]es fit the shape of their container. |
|- | |- | ||
| − | | style="height:20px; width: | + | | style="height:20px; width:200px; text-align:center;" |[[Convection]] happens most easily in [[gas]]es. |
| + | |- | ||
| + | |rowspan="3"|[[Particle]]s are spread apart. | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Gas]]es can be [[compressed]] into a smaller [[Volume (Space)|volume]]. | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Sound]] passes through [[gas]]es slower than [[liquid]]s and [[solid]]s. | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |[[Thermal Conduction]] is very poor in a [[gas]]es. | ||
|} | |} | ||
| − | + | ====Why isn't everything a gas?==== | |
| − | + | : When [[particle]]s are near each other, they tend to stick together. This is due to [[force]]s acting between [[adjacent]] [[particle]]s. When two [[particle]]s are near one another they are [[attracted]] together. This causes those [[particle]]s to come together. Without this [[force]] of [[attraction]] between particles they would not stick together and there would be no [[solid]] or [[liquid]] '''states'''. | |
| − | + | : What determines if a [[substance]] is a [[solid]], [[liquid]] or [[gas]] at [[Room Temperature|room temperature]] is how big that [[force]] of [[attraction]] is. | |
| + | ====Why Solids and Liquids exist==== | ||
| + | : Different [[substance]]s have a different [[force]] of [[attraction]] between the [[adjacent]] [[particle]]s. | ||
| + | : Silicon dioxide (sand and glass) is [[solid]] at [[Room Temperature|room temperature]] because there is a strong [[force]] of [[attraction]] between [[adjacent]] [[molecule]]s. | ||
| + | : [[Water]] is a [[liquid]] at [[Room Temperature|room temperature]] because the [[force]] of [[attraction]] is not great enough to hold the [[molecule]]s in position. However, it is great enough to keep them together. | ||
| + | : [[Oxygen]] is a gas at [[Room Temperature|room temperature]] because there is a very weak [[force]] of [[attraction]] between [[adjacent]] [[molecule]]s. | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782945970/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945970&linkCode=as2&tag=nrjc-21&linkId=a120d24dcc7cc7a58192069a3aafc1d2 ''States of matter, pages 106, 107, 110-113, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945598/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945598&linkCode=as2&tag=nrjc-21&linkId=ad276ad49df77ab4b40ab4fd0fe10313 ''States of matter, pages 121, 122, 193, 195, GCSE Combined Science; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851354/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851354&linkCode=as2&tag=nrjc-21&linkId=9012a0d354024419214fb3ad5ac44ba0 ''States of matter, pages 164-5, 323, GCSE Combined Science Trilogy 1, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945571/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945571&linkCode=as2&tag=nrjc-21&linkId=9e29fad914244909903e5e93f8a01d244 ''States of matter, pages 36, 37, GCSE Chemistry; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''States of matter, pages 36-37, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294558X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294558X&linkCode=as2&tag=nrjc-21&linkId=f0dfb66dafcb0c6e9449e7b1a4ae1ac428 ''States of matter, pages 38-40, GCSE Physics; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''States of matter, pages 49-50, GCSE Chemistry, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''States of matter, pages 56-7, 59, 68-9, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/019835939X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=019835939X&linkCode=as2&tag=nrjc-21&linkId=57e96876985fc39b1a3d8a3e3dc238b6 ''States of matter, pages 78-85, GCSE Physics; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158770/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158770&linkCode=as2&tag=nrjc-21&linkId=ec31595e720e1529e49876c3866fff6e ''States of matter, pages 84, 100-1, GCSE Physics; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782946403/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782946403&linkCode=as2&tag=nrjc-21&linkId=32a0abb60dff015b15b50e9b1d7b4644 ''States of matter, pages 96, 97, 100-103, GCSE Combined Science Trilogy; Physics, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294639X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294639X&linkCode=as2&tag=nrjc-21&linkId=51599bb45a2bfaf7c1b6a978b2ca2616 ''States of matter, pages 97, 101, GCSE Combined Science Trilogy; Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''States of matter, pages 99, 103, GCSE Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851354/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851354&linkCode=as2&tag=nrjc-21&linkId=9012a0d354024419214fb3ad5ac44ba0 ''States of matter; changes of, pages 324-5, GCSE Combined Science Trilogy 1, Hodder, AQA ''] | ||
| + | |||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782945741/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945741&linkCode=as2&tag=nrjc-21&linkId=30da4f2178da182547b62a7329d13b57 ''Sates of matter, pages 97, 98, 201, 203, GCSE Combined Science; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120223/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120223&linkCode=as2&tag=nrjc-21&linkId=068ecf40278c32406a7f1c6e66751417 ''States of matter, page 182, GCSE Physics, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''States of matter, pages 2-3, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948163/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948163&linkCode=as2&tag=nrjc-21&linkId=0fdbfd5dd397d6e24a9dfb250f08587f ''States of matter, pages 299, 300, GCSE Physics, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945733/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945733&linkCode=as2&tag=nrjc-21&linkId=2a2dbec9db6bf5766c0458d908fa0a52 ''States of matter, pages 94, 96, GCSE Physics; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948147/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948147&linkCode=as2&tag=nrjc-21&linkId=f63dcd8345f4e49c717b39a228a36c7c ''States of matter, pages 95-99, GCSE Chemistry, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948163/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948163&linkCode=as2&tag=nrjc-21&linkId=0fdbfd5dd397d6e24a9dfb250f08587f ''States of matter; changing state, pages 302, 303, GCSE Physics, CGP, Edexcel ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945679/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945679&linkCode=as2&tag=nrjc-21&linkId=a2db42f7b4bdf10cafaafa3bb9120940 ''States of matter, page 12, Gateway GCSE Chemistry; The Revision Guide, CGP, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945687/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945687&linkCode=as2&tag=nrjc-21&linkId=9a598e52189317a20311d7a632747bc9 ''States of matter, page 14, Gateway GCSE Physics; The Revision Guide, CGP, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''States of matter, pages 18-21, 76-77, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945695/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945695&linkCode=as2&tag=nrjc-21&linkId=ceafcc80bcad6b6754ee97a0c7ceea53 ''States of matter, pages 82, 152, 154, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR ''] | ||
Latest revision as of 10:53, 24 February 2021
Contents
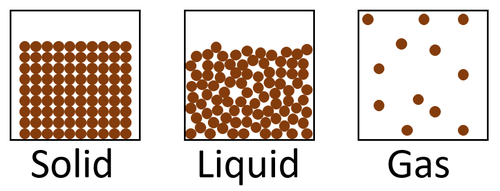
Key Stage 2
Meaning
State of matter means whether a material is solid, liquid or gas.
About States of Matter
- Materials can be solid, liquid or gas.
- The state of matter can be changed by heating or cooling the material.
- Heating can turn a solid into a liquid. This is called melting.
- Heating can turn a liquid to a gas. This is called evaporating.
- Cooling can turn a gas into a liquid. This is called condensing.
- Cooling can turn a liquid into solid. This is called freezing.
| Brick is a solid material. | Water is a liquid material. | Inside the balloon is a gas called helium. |
Key Stage 3
Meaning
State of matter means whether a material is solid, liquid or gas.
About States of Matter
- Materials can be solid, liquid or gas.
- The state of matter can be changed by heating or cooling the material.
- Heating can turn a solid into a liquid by melting or it can turn a solid straight into a gas by subliming.
- Heating can turn a liquid to a gas. This is called evaporating.
- Cooling can turn a gas into a liquid by condensing or it can turn a gas into a solid by depositing.
- Cooling can turn a liquid into solid. This is called freezing.
| This diagram shows the 3 states of matter in the particle model. |
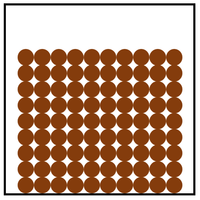
Properties of the States of Matter
| Solid | Liquid | Gas |
| Cannot be compressed. | Cannot be compressed. | Can be compressed. |
| Does not flow. | Can flow. | Can flow. |
| Holds its shape. | Fits the shape of the container. | Fits the size and shape of the container. |
Key Stage 4
Meaning
State of matter means whether a material is solid, liquid or gas.
About States of Matter
- Materials can be solid, liquid or gas.
- The state of matter can be altered by changing the temperature of the material or changing the pressure on the material.
- The properties of solids, liquids and gases can be explained by the way the particles inside those substances behave.
| Particle Diagram | Particle Arrangement | Property |
| Particles are in fixed positions. | Solids hold their shape. | |
| Convection cannot happen in solids. | ||
| Particles are very close together. | Solids cannot be compressed. | |
| Sound passes through solids faster than liquids and gases. | ||
| Particles vibrate. | ||
| Thermal Conduction happens best in solids. |
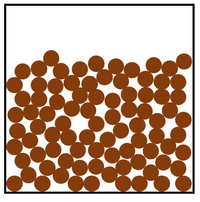
| Particle Diagram | Particle Arrangement | Property |
| Particles can slide past each other. | Liquids can be poured. | |
| Liquids fit the shape of their container. | ||
| Convection happens in solids. | ||
| Particles are close together. | Liquids cannot be compressed. | |
| Sound passes through liquids faster than gases. | ||
| Thermal Conduction happens in liquids but not as well as in solids. |
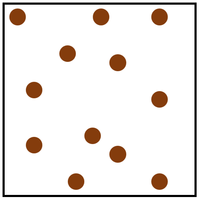
| Particle Diagram | Particle Arrangement | Property |
| Particles are free to move in all directions. | Gases fit the size of their container. | |
| Gases fit the shape of their container. | ||
| Convection happens most easily in gases. | ||
| Particles are spread apart. | Gases can be compressed into a smaller volume. | |
| Sound passes through gases slower than liquids and solids. | ||
| Thermal Conduction is very poor in a gases. |
Why isn't everything a gas?
- When particles are near each other, they tend to stick together. This is due to forces acting between adjacent particles. When two particles are near one another they are attracted together. This causes those particles to come together. Without this force of attraction between particles they would not stick together and there would be no solid or liquid states.
- What determines if a substance is a solid, liquid or gas at room temperature is how big that force of attraction is.
Why Solids and Liquids exist
- Different substances have a different force of attraction between the adjacent particles.
- Silicon dioxide (sand and glass) is solid at room temperature because there is a strong force of attraction between adjacent molecules.
- Water is a liquid at room temperature because the force of attraction is not great enough to hold the molecules in position. However, it is great enough to keep them together.
- Oxygen is a gas at room temperature because there is a very weak force of attraction between adjacent molecules.
References
AQA
- States of matter, pages 106, 107, 110-113, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA
- States of matter, pages 121, 122, 193, 195, GCSE Combined Science; The Revision Guide, CGP, AQA
- States of matter, pages 164-5, 323, GCSE Combined Science Trilogy 1, Hodder, AQA
- States of matter, pages 36, 37, GCSE Chemistry; The Revision Guide, CGP, AQA
- States of matter, pages 36-37, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- States of matter, pages 38-40, GCSE Physics; The Revision Guide, CGP, AQA
- States of matter, pages 49-50, GCSE Chemistry, Hodder, AQA
- States of matter, pages 56-7, 59, 68-9, GCSE Chemistry; Student Book, Collins, AQA
- States of matter, pages 78-85, GCSE Physics; Third Edition, Oxford University Press, AQA
- States of matter, pages 84, 100-1, GCSE Physics; Student Book, Collins, AQA
- States of matter, pages 96, 97, 100-103, GCSE Combined Science Trilogy; Physics, CGP, AQA
- States of matter, pages 97, 101, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- States of matter, pages 99, 103, GCSE Chemistry, CGP, AQA
- States of matter; changes of, pages 324-5, GCSE Combined Science Trilogy 1, Hodder, AQA
Edexcel
- Sates of matter, pages 97, 98, 201, 203, GCSE Combined Science; The Revision Guide, CGP, Edexcel
- States of matter, page 182, GCSE Physics, Pearson Edexcel
- States of matter, pages 2-3, GCSE Chemistry, Pearson, Edexcel
- States of matter, pages 299, 300, GCSE Physics, CGP, Edexcel
- States of matter, pages 94, 96, GCSE Physics; The Revision Guide, CGP, Edexcel
- States of matter, pages 95-99, GCSE Chemistry, CGP, Edexcel
- States of matter; changing state, pages 302, 303, GCSE Physics, CGP, Edexcel
OCR
- States of matter, page 12, Gateway GCSE Chemistry; The Revision Guide, CGP, OCR
- States of matter, page 14, Gateway GCSE Physics; The Revision Guide, CGP, OCR
- States of matter, pages 18-21, 76-77, Gateway GCSE Chemistry, Oxford, OCR
- States of matter, pages 82, 152, 154, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR