Difference between revisions of "Element"
| Line 1: | Line 1: | ||
==Key Stage 3== | ==Key Stage 3== | ||
===Meaning=== | ===Meaning=== | ||
| + | [[File:ElementTile.png|right|400px|thumb|[[Element]]s are show on the [[Periodic Table]] with the [[Chemical Symbol]], [[Atomic Number]] and [[Relative Atomic Mass]].]] | ||
An '''element''' is a [[material]] made of only one type of [[atom]]. | An '''element''' is a [[material]] made of only one type of [[atom]]. | ||
Revision as of 21:19, 5 December 2018
Key Stage 3
Meaning
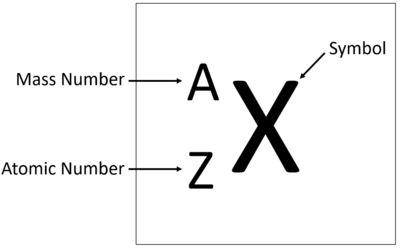
Elements are show on the Periodic Table with the Chemical Symbol, Atomic Number and Relative Atomic Mass.
An element is a material made of only one type of atom.
About Elements
- There are over 100 different elements and they are all shown on the Periodic Table.
- An element can be as little as one atom or could be trillions of identical atoms.
- If there are any other types of atom in an object it is not an element.
| Gold is an element made of Gold Atoms. | These lumps of Sulphur are made of only Sulphur atoms. | This piece of Boron is made of only Boron atoms. | A diamond is made of only Carbon atoms making it pure carbon. |
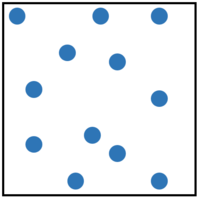
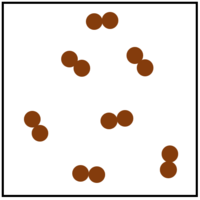
- Elements can be represented in a particle diagram by showing only one colour or shade to represent that atoms.
| This is a solid element. An example would be gold. | This is a liquid element. An example would be mercury. | This is a gaseous element. An example would be helium. | This gaseous element is made of molecules of the element. An example would be oxygen. |
Key Stage 4
Meaning
An element is a material made of atoms that all have the same atomic number.
About Elements
- Atoms of the same element all have the same chemical properties.
- There are several isotopes of each element which have slightly different physical properties such as density and melting point.