Difference between revisions of "Electron Orbital"
| Line 1: | Line 1: | ||
| + | ==Key Stage 3== | ||
| + | ===Meaning=== | ||
| + | '''Electron shells''' are the places around a [[nucleus]] where an [[electron]] can [[orbit]] the [[nucleus]]. | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:SodiumShells.png|center|400px]] | ||
| + | |- | ||
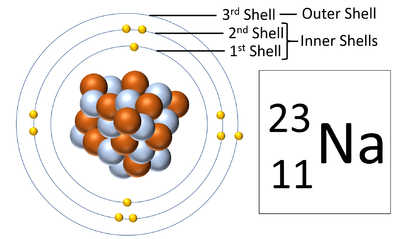
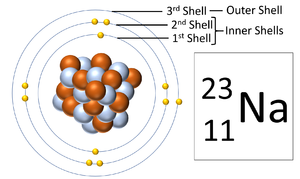
| + | | style="height:20px; width:200px; text-align:center;" |A [[diagram]] of a [[Sodium]] [[atom]] shown the '''electron shells'''. | ||
| + | |} | ||
| + | |||
| + | ===About Electron Shells=== | ||
| + | : The number of '''electron shells''' is shown by the [[period]] on the [[Periodic Table]]. | ||
| + | : The number of [[electron]]s in the [[Outer Shell|outer shell]] determines the [[Chemical Property|chemical properties]] of the [[element]]. | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:ElectronShells.png|center|800px]] | ||
| + | |- | ||
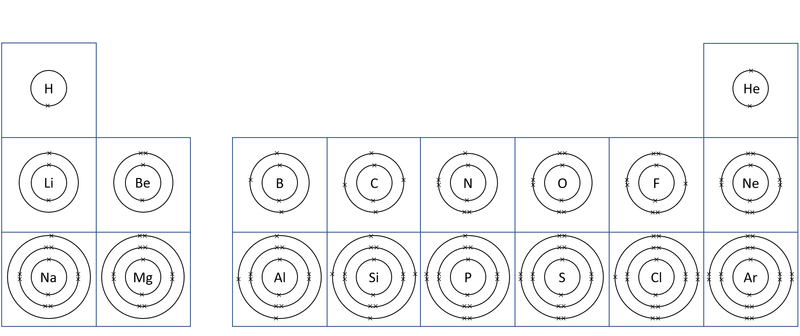
| + | | style="height:20px; width:200px; text-align:center;" |A [[diagram]] of the first 20 [[element]]s in the [[Periodic Table]] showing the '''electron shells'''. | ||
| + | |} | ||
| + | |||
==Key Stage 4== | ==Key Stage 4== | ||
===Meaning=== | ===Meaning=== | ||
Revision as of 12:29, 6 December 2018
Contents
Key Stage 3
Meaning
Electron shells are the places around a nucleus where an electron can orbit the nucleus.
| A diagram of a Sodium atom shown the electron shells. |
About Electron Shells
- The number of electron shells is shown by the period on the Periodic Table.
- The number of electrons in the outer shell determines the chemical properties of the element.
| A diagram of the first 20 elements in the Periodic Table showing the electron shells. |
Key Stage 4
Meaning
An electron orbital, also known as an electron shell, are the locations where electrons orbit the nucleus of atoms.
About Electron Orbitals
- Each electron orbital only holds a certain number of electrons.
- These orbitals and the number of electrons in an atom determine the chemistry of an element.
- The number of electron orbitals determines the Period on the Periodic Table.
- The number of electrons in the last orbital (Outer Shell) determines the Group on the Periodic Table.
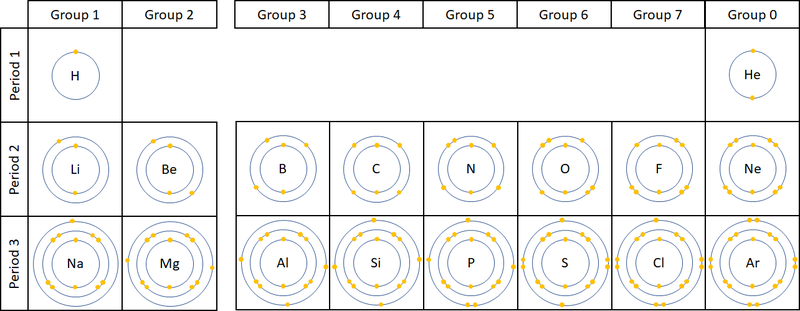
| A diagram showing the electron shells and electrons in the first 20 elements on the Periodic Table. NB Group 0 used to be called Group 8 but this caused confusion because most elements in Group 8 have 8 electrons in their Outer Shell but Helium only has 2, so it was renamed Group 0. |
- Atoms in the same group have similar chemical properties because they all have the same number of electrons in their Outer Shell.