Difference between revisions of "Chemical Bond"
(→Examples) |
(→Examples) |
||
| Line 35: | Line 35: | ||
{| class="wikitable" | {| class="wikitable" | ||
| − | |+ | + | |+ Metallic Bonds |
|- | |- | ||
|[[File:MagnesiumMetallicBond.png|center|300px]] | |[[File:MagnesiumMetallicBond.png|center|300px]] | ||
Revision as of 16:45, 20 December 2018
Key Stage 4
Meaning
A chemical bond is a force of attraction holding the atoms inside a molecule together.
About Chemical Bonds
There are three types of chemical bond you should know:
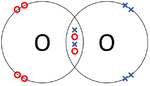
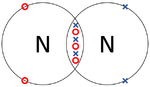
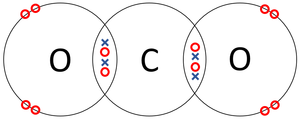
- Covalent Bonds - In which atoms share electrons with one another.
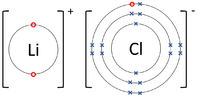
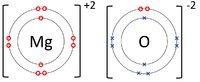
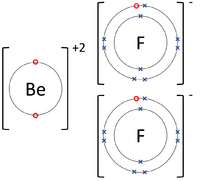
- Ionic Bonds - In which electrons are transferred from one atom to another.
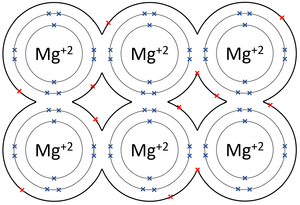
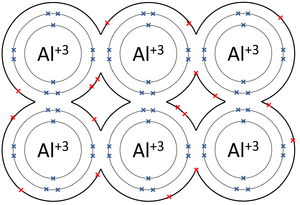
- Metallic Bonds - In which some electrons move freely between atoms creating lattice of positively charged ions surrounded by a sea of delocalised electrons (free electrons).
Examples
| The two Oxygen atoms each share two of their electrons with one another. | The two Nitrogen atoms each share three of their electrons with one another. | Each Oxygen shares two of its electrons with the Carbon atom while the Carbon atom shares two electrons with each Oxygen atom. |
| The Lithium atom donates an electron from its outer shell to the outer shell of the Fluorine atom. | The Magnesium atom donates two electrons from its outer shell to the outer shell of the Oxygen atom. | The Beryllium atom donates an electron from its outer shell to the outer shell of each Fluorine atom. |
| The outer shells of the Magnesium atoms overlap allowing the two electrons in each outer shell to move freely between atoms. | The outer shells of the Aluminium atoms overlap allowing the three electrons in each outer shell to move freely between atoms. |