Difference between revisions of "Metal Ion"
(Created page with "==Key Stage 4== ===Meaning=== Metal Ions are positive ions found in ionic compounds and Giant Metallic Structure|giant metallic struc...") |
|||
| Line 5: | Line 5: | ||
===About Metal Ions=== | ===About Metal Ions=== | ||
: '''Metal ions''' are formed when [[metal]] [[element]]s lose their [[electron]]s to form [[Positive Ion|positive ions]]. | : '''Metal ions''' are formed when [[metal]] [[element]]s lose their [[electron]]s to form [[Positive Ion|positive ions]]. | ||
| − | The [[charge]] on a '''metal ion''' may be determined by the [[Group]]. | + | The [[Electrical Charge|charge]] on a '''metal ion''' may be determined by the [[Group]]. |
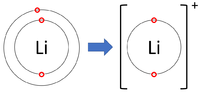
*[[Group 1]] [[Element]]s all form +1 [[ion]]s; Li<sup>+1</sup>, Na<sup>+1</sup>, K<sup>+1</sup> | *[[Group 1]] [[Element]]s all form +1 [[ion]]s; Li<sup>+1</sup>, Na<sup>+1</sup>, K<sup>+1</sup> | ||
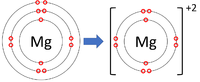
*[[Group 2]] [[Element]]s all form +2 [[ion]]s; Be<sup>+2</sup>, Mg<sup>+2</sup>, Ca<sup>+2</sup> | *[[Group 2]] [[Element]]s all form +2 [[ion]]s; Be<sup>+2</sup>, Mg<sup>+2</sup>, Ca<sup>+2</sup> | ||
Revision as of 11:00, 2 February 2019
Key Stage 4
Meaning
Metal Ions are positive ions found in ionic compounds and giant metallic structures.
About Metal Ions
- Metal ions are formed when metal elements lose their electrons to form positive ions.
The charge on a metal ion may be determined by the Group.
- Group 1 Elements all form +1 ions; Li+1, Na+1, K+1
- Group 2 Elements all form +2 ions; Be+2, Mg+2, Ca+2
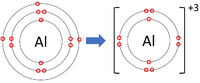
- Group 3 Elements all form +3 ions; Al+3
Transition Metal Elements can form different ions which are shown by Roman Numerals; Iron can form Fe(II) which is Fe+2 or Fe(III) is Fe+3, Manganese can form Mn(II) which is Mn+2 or Mn(IV) which is Mn+4.
Examples
| Lithium forms +1 ions. | Magnesium forms +2 ions. | Aluminium forms +3 ions. |