Difference between revisions of "Endothermic"
(Created page with "==Key Stage 3== ===Meaning=== An endothermic process is one takes in energy. This usually causes surroundings to decrease in temperature. ===About Exothermic Proc...") |
|||
| (5 intermediate revisions by 2 users not shown) | |||
| Line 13: | Line 13: | ||
: [[Melting]], [[Evaporating]] and [[Subliming]] are [[endothermic]] changes because they need [[energy]] to happen. The [[material]] has more [[energy]] at the end than it did the beginning. However, there is usually no decrease in [[temperature]] because the [[material]] is usually being heated to [[State Change|change state]]. | : [[Melting]], [[Evaporating]] and [[Subliming]] are [[endothermic]] changes because they need [[energy]] to happen. The [[material]] has more [[energy]] at the end than it did the beginning. However, there is usually no decrease in [[temperature]] because the [[material]] is usually being heated to [[State Change|change state]]. | ||
: [[Dissolve|Dissolving]] is an [[endothermic]] process as [[energy]] is needed to break the [[bond]]s in the [[solid]] [[solute]] to [[dissolve]]. This causes the [[solution]] to decrease in [[temperature]] while the [[solute]] is [[dissolve|dissolving]]. | : [[Dissolve|Dissolving]] is an [[endothermic]] process as [[energy]] is needed to break the [[bond]]s in the [[solid]] [[solute]] to [[dissolve]]. This causes the [[solution]] to decrease in [[temperature]] while the [[solute]] is [[dissolve|dissolving]]. | ||
| + | |||
| + | ==Key Stage 4== | ||
| + | ===Meaning=== | ||
| + | An [[endothermic]] process is one takes in [[energy]]. This usually causes surroundings to decrease in [[temperature]]. | ||
| + | |||
| + | ===About Endothermic Processes=== | ||
| + | ====Foundation==== | ||
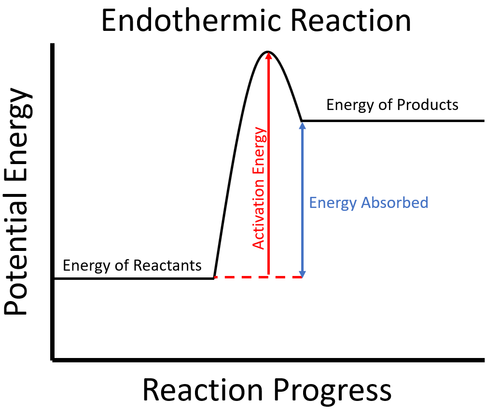
| + | : [[Endothermic]] [[Chemical Reaction|reactions]] usually require [[energy]] to begin. This is called the [[Activation Energy|activation energy]]. | ||
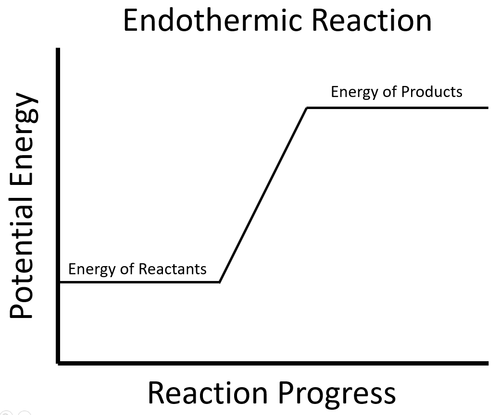
| + | : In an [[endothermic]] process the [[Potential Energy|potential energy]] stored in the [[reactant]]s is less than the [[Potential Energy|potential energy]] stored in the [[product]]s. | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:EndothermicSketchGraphKS4.png|center|500px]] | ||
| + | |- | ||
| + | | style="height:20px; width:500px; text-align:center;" |To start the [[Chemical Reaction|reaction]] an [[Activation Energy|activation energy]] is needed. This is usually achieved by initially [[heating]] the [[reactant]]s. Once the [[Chemical Reaction|reaction]] starts, since the [[energy]] stored in the [[reactant]]s less than the [[energy]] stored in the [[product]]s, this means the [[Chemical Reaction|reaction]] must take in [[energy]] from the environment which causes the surroundings to decrease in [[temperature]]. | ||
| + | |} | ||
| + | ====Higher==== | ||
| + | : In a [[Chemical Reaction|chemical reaction]] [[energy]] is needed to break the [[Chemical Bond|chemical bonds]] holding the [[atom]]s together. | ||
| + | : In an [[endothermic]] [[Chemical Reaction|reaction]] [[energy]] is then [[Absorb (Physics)|absorbed]] as [[atom]]s form new [[Chemical Bond|chemical bonds]]. | ||
| + | : In an [[endothermic]] [[Chemical Reaction|reaction]] more [[energy]] is taken in breaking the [[Chemical Bond|bonds]] in the [[reactant]]s than is released in the formation of new [[Chemical Bond|bonds]] in the [[product]]s. So there is a net input of [[energy]]. | ||
| + | |||
| + | ==Beyond the Curriculum== | ||
| + | {{#ev:youtube|https://www.youtube.com/watch?v=hVh-bpAv4_E}} | ||
| + | |||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/0008158754/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158754&linkCode=as2&tag=nrjc-21&linkId=27ad53b0283feeff7fc5ae04a9e205f262 ''Endothermic, pages 56, 58, GCSE Biology; Student Book, Collins, AQA ''] | ||
| + | |||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782948120/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948120&linkCode=as2&tag=nrjc-21&linkId=dedef775c6a43dbb0a609441525adac0 ''Endothermic reactions, page 198, GCSE Biology, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945741/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945741&linkCode=as2&tag=nrjc-21&linkId=30da4f2178da182547b62a7329d13b57 ''Endothermic reactions, pages 134, 136, GCSE Combined Science; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Endothermic reactions, pages 138, 144-145, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948147/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948147&linkCode=as2&tag=nrjc-21&linkId=f63dcd8345f4e49c717b39a228a36c7c ''Endothermic reactions, pages 243, 244, 248-250, GCSE Chemistry, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Endothermic reactions, pages 252, 258-259, GCSE Combined Science, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945725/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945725&linkCode=as2&tag=nrjc-21&linkId=694be7494de75af3349537d34e13f7f0 ''Endothermic reactions, pages 83, 85, GCSE Chemistry; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120207/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120207&linkCode=as2&tag=nrjc-21&linkId=22455ff53961978667722edaa64c0be5 ''Endothermic reactions, photosynthesis, page 124, GCSE Biology, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Endothermic reactions; equilibrium, page 239, GCSE Combined Science, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Endothermic reactions; equilibrium, page 95, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Endothermic reactions; photosynthesis, page 86, GCSE Combined Science, Pearson Edexcel ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945660/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945660&linkCode=as2&tag=nrjc-21&linkId=83aa4500ad7759e7f401a1c5ba5df758 ''Endothermic reactions, page 25, Gateway GCSE Biology; The Revision Guide, CGP, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359810/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359810&linkCode=as2&tag=nrjc-21&linkId=d768d99f1a06f7c12fab40e5aef85a55 ''Endothermic reactions, page 46, Gateway GCSE Biology, Oxford, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Endothermic reactions, pages 102-103, 105, 186, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945695/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945695&linkCode=as2&tag=nrjc-21&linkId=ceafcc80bcad6b6754ee97a0c7ceea53 ''Endothermic reactions, pages 21, 111, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945679/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945679&linkCode=as2&tag=nrjc-21&linkId=a2db42f7b4bdf10cafaafa3bb9120940 ''Endothermic reactions, pages 41, 42, Gateway GCSE Chemistry; The Revision Guide, CGP, OCR ''] | ||
Latest revision as of 14:51, 6 December 2019
Contents
Key Stage 3
Meaning
An endothermic process is one takes in energy. This usually causes surroundings to decrease in temperature.
About Exothermic Processes
- A few chemical reactions are endothermic with means they need energy from the environment and this is observed by a decrease in temperature.
| The energy stored in the reactants less than the energy stored in the products. This means the reaction must take in energy from the environment which causes the surroundings to decrease in temperature. |
- Melting, Evaporating and Subliming are endothermic changes because they need energy to happen. The material has more energy at the end than it did the beginning. However, there is usually no decrease in temperature because the material is usually being heated to change state.
- Dissolving is an endothermic process as energy is needed to break the bonds in the solid solute to dissolve. This causes the solution to decrease in temperature while the solute is dissolving.
Key Stage 4
Meaning
An endothermic process is one takes in energy. This usually causes surroundings to decrease in temperature.
About Endothermic Processes
Foundation
- Endothermic reactions usually require energy to begin. This is called the activation energy.
- In an endothermic process the potential energy stored in the reactants is less than the potential energy stored in the products.
| To start the reaction an activation energy is needed. This is usually achieved by initially heating the reactants. Once the reaction starts, since the energy stored in the reactants less than the energy stored in the products, this means the reaction must take in energy from the environment which causes the surroundings to decrease in temperature. |
Higher
- In a chemical reaction energy is needed to break the chemical bonds holding the atoms together.
- In an endothermic reaction energy is then absorbed as atoms form new chemical bonds.
- In an endothermic reaction more energy is taken in breaking the bonds in the reactants than is released in the formation of new bonds in the products. So there is a net input of energy.
Beyond the Curriculum
References
AQA
Edexcel
- Endothermic reactions, page 198, GCSE Biology, CGP, Edexcel
- Endothermic reactions, pages 134, 136, GCSE Combined Science; The Revision Guide, CGP, Edexcel
- Endothermic reactions, pages 138, 144-145, GCSE Chemistry, Pearson, Edexcel
- Endothermic reactions, pages 243, 244, 248-250, GCSE Chemistry, CGP, Edexcel
- Endothermic reactions, pages 252, 258-259, GCSE Combined Science, Pearson Edexcel
- Endothermic reactions, pages 83, 85, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Endothermic reactions, photosynthesis, page 124, GCSE Biology, Pearson, Edexcel
- Endothermic reactions; equilibrium, page 239, GCSE Combined Science, Pearson Edexcel
- Endothermic reactions; equilibrium, page 95, GCSE Chemistry, Pearson, Edexcel
- Endothermic reactions; photosynthesis, page 86, GCSE Combined Science, Pearson Edexcel
OCR
- Endothermic reactions, page 25, Gateway GCSE Biology; The Revision Guide, CGP, OCR
- Endothermic reactions, page 46, Gateway GCSE Biology, Oxford, OCR
- Endothermic reactions, pages 102-103, 105, 186, Gateway GCSE Chemistry, Oxford, OCR
- Endothermic reactions, pages 21, 111, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR
- Endothermic reactions, pages 41, 42, Gateway GCSE Chemistry; The Revision Guide, CGP, OCR