Difference between revisions of "Metal Ion"
| Line 24: | Line 24: | ||
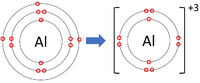
| style="height:20px; width:200px; text-align:center;" |[[Aluminium]] forms +3 [[ion]]s. | | style="height:20px; width:200px; text-align:center;" |[[Aluminium]] forms +3 [[ion]]s. | ||
|} | |} | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782945571/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945571&linkCode=as2&tag=nrjc-21&linkId=9e29fad914244909903e5e93f8a01d255 ''Metal ions, pages 89, 90, GCSE Chemistry; The Revision Guide, CGP, AQA ''] | ||
Revision as of 15:31, 8 November 2019
Key Stage 4
Meaning
Metal Ions are positive ions found in ionic compounds and giant metallic structures.
About Metal Ions
- Metal ions are formed when metal elements lose their electrons to form positive ions.
The charge on a metal ion may be determined by the Group.
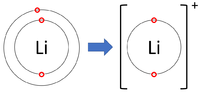
- Group 1 Elements all form +1 ions; Li+1, Na+1, K+1
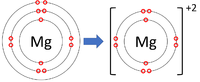
- Group 2 Elements all form +2 ions; Be+2, Mg+2, Ca+2
- Group 3 Elements all form +3 ions; Al+3
Transition Metal Elements can form different ions which are shown by Roman Numerals; Iron can form Fe (II) which is Fe +2 or Fe (III) is Fe+3, Manganese can form Mn (II) which is Mn+2 or Mn (IV) which is Mn+4.
Examples
| Lithium forms +1 ions. | Magnesium forms +2 ions. | Aluminium forms +3 ions. |