Difference between revisions of "Nuclear Model"
| Line 12: | Line 12: | ||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
| − | | style="height:20px; width: | + | | style="height:20px; width:300px; text-align:center;" |'''Observation''' |
| − | | style="height:20px; width: | + | | style="height:20px; width:300px; text-align:center;" |'''Conclusion''' |
|- | |- | ||
| − | | style="height:20px; width: | + | | style="height:20px; width:300px; text-align:center;" |Most of the [[Alpha Particle|alpha particle]]s pass straight through the foil. |
| − | | style="height:20px; width: | + | | style="height:20px; width:300px; text-align:center;" |The [[atom]] must be mostly empty space. |
|- | |- | ||
| − | | style="height:20px; width: | + | | style="height:20px; width:300px; text-align:center;" |Some of the [[Alpha Particle|alpha particle]]s were deflected by a small angle. |
| − | | style="height:20px; width: | + | | style="height:20px; width:300px; text-align:center;" |The [[mass]] of the [[atom]] must be concentrated in an extremely small [[Volume (Space)|volume]] in the centre. |
|- | |- | ||
| − | | style="height:20px; width: | + | | style="height:20px; width:300px; text-align:center;" |A very small number of [[Alpha Particle|alpha particle]]s came back in the direction of the detector. (Deflected more than 90°.) |
| − | | style="height:20px; width: | + | | style="height:20px; width:300px; text-align:center;" |The centre of an [[atom]] must have a strong positive charge. |
The [[electron]]s must not be in the centre of the [[atom]], they must be [[orbit]]ing the [[nucleus]]. | The [[electron]]s must not be in the centre of the [[atom]], they must be [[orbit]]ing the [[nucleus]]. | ||
|} | |} | ||
| − | |||
| − | |||
Revision as of 12:05, 24 November 2018
Key Stage 4
Meaning
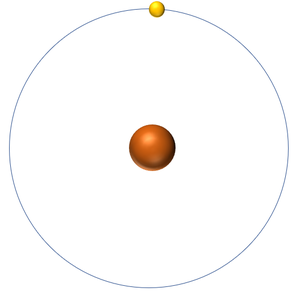
The Nuclear Model is a model of the atom which suggests there is a positively charged nucleus in the centre of an atom with electrons orbiting around the nucleus.
About the Nuclear Model
- The Nuclear Model was first proposed by Ernest Rutherford after he successfully disproved the Plum Pudding Model of the atom using his alpha scattering experiment.
- In the Nuclear Model most of the atom is empty space. The majority of the mass of the atom is concentrated in a small central nucleus. This central nucleus has a strong positive charge while negatively charged electrons orbit the nucleus.
Evidence for the Nuclear Model
- In Rutherford's Alpha Scattering Experiment several observations about the path of alpha particles through a thin sheet of Gold foil that led to the development of the Nuclear Model:
| Observation | Conclusion |
| Most of the alpha particles pass straight through the foil. | The atom must be mostly empty space. |
| Some of the alpha particles were deflected by a small angle. | The mass of the atom must be concentrated in an extremely small volume in the centre. |
| A very small number of alpha particles came back in the direction of the detector. (Deflected more than 90°.) | The centre of an atom must have a strong positive charge.
The electrons must not be in the centre of the atom, they must be orbiting the nucleus. |