Difference between revisions of "Outer Shell"
| Line 7: | Line 7: | ||
: The '''outer shell''' is the only [[Electron Orbital|electron orbital]] that is affected in [[Chemical Reaction|chemical reactions]]. | : The '''outer shell''' is the only [[Electron Orbital|electron orbital]] that is affected in [[Chemical Reaction|chemical reactions]]. | ||
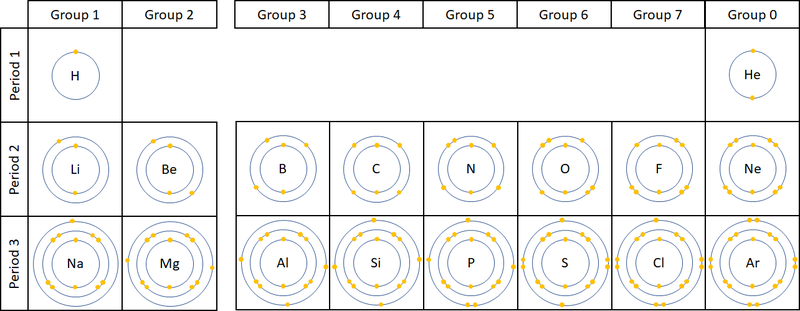
: The number of [[electron]]s in the last '''Outer Shell''' determines the [[Group (Chemistry)|Group]] on the [[Periodic Table]]. | : The number of [[electron]]s in the last '''Outer Shell''' determines the [[Group (Chemistry)|Group]] on the [[Periodic Table]]. | ||
| + | : The [[Outer Shell|outer shell]] is the highest [[Energy Level|energy level]] in an [[atom]]. | ||
| + | : [[Electron]]s in the [[Outer Shell|outer shell]] may gain enough [[energy]] from [[collision]]s or from [[Electromagnetic Radiation|electromagnetic radiation]] to leave the [[atom]] producing a free [[electron]] and turning the [[atom]] into an [[ion]]. | ||
| + | : [[Ionising Radiation]] can cause [[electron]]s to leave the [[Outer Shell|outer shell]] leaving behind a [[Positive Ion|positively charged ion]]. | ||
| + | |||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
Latest revision as of 09:15, 7 March 2019
Key Stage 3
Meaning
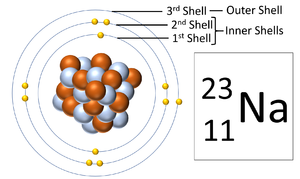
A diagram showing the three electron shells of a Sodium atom. The outer shell is the electron orbital furthest from the nucleus.
The outer shell is the electron orbital furthest from the nucleus of an atom.
About the Outer Shell
- The outer shell is the only electron orbital that is affected in chemical reactions.
- The number of electrons in the last Outer Shell determines the Group on the Periodic Table.
- The outer shell is the highest energy level in an atom.
- Electrons in the outer shell may gain enough energy from collisions or from electromagnetic radiation to leave the atom producing a free electron and turning the atom into an ion.
- Ionising Radiation can cause electrons to leave the outer shell leaving behind a positively charged ion.
| This diagram shows that the number of electrons in the outer shell determines which group the elements have been placed into in the Periodic Table. NB Group 0 used to be called Group 8 but this caused confusion because most elements in Group 8 have 8 electrons in their Outer Shell but Helium only has 2, so it was renamed Group 0. |