Key Stage 4
Meaning
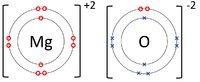
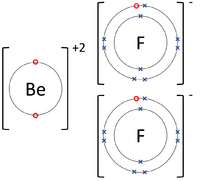
A dot and cross diagram is a diagram used to show how electrons from the outer shells of atoms are shared or transferred in a chemical bond.
About Dot and Cross Diagrams
- Dot and cross diagrams can be used to represent covalent bonds and ionic bonds.
Examples
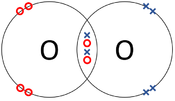
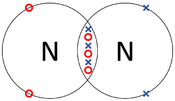
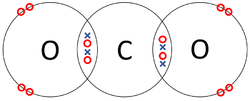
| The two Oxygen atoms each share two of their electrons with one another. | The two Nitrogen atoms each share three of their electrons with one another. | Each Oxygen shares two of its electrons with the Carbon atom while the Carbon atom shares two electrons with each Oxygen atom. |
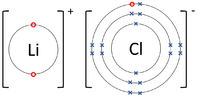
| The Lithium atom donates an electron from its outer shell to the outer shell of the Fluorine atom. | The Magnesium atom donates two electrons from its outer shell to the outer shell of the Oxygen atom. | The Beryllium atom donates two electrons from its outer shell to the outer shells of each Fluorine atom. |