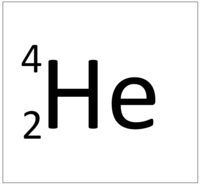Contents
Key Stage 3
Meaning
An atom is a very small particle made of protons, neutrons and electrons that can join with other atoms to make molecules.
About Atoms in The Dalton Model
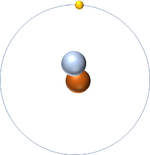
- In The Dalton Model atoms are shown as ball shaped particles. This makes it easier to draw diagrams of molecules.
| A picture of The Dalton Model of an atom. |
About Atoms beyond The Dalton Model
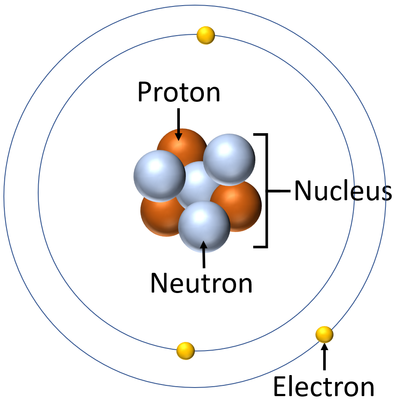
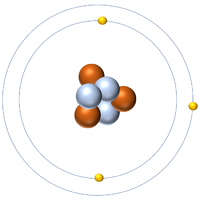
- Atoms are made of three smaller particles; the proton, neutron and electron.
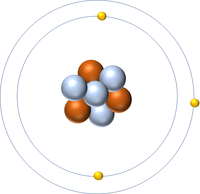
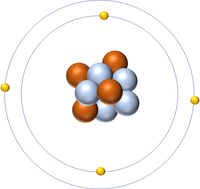
- Protons and neutrons are found in the nucleus at the centre of an atom. Electrons are found orbiting the nucleus in 'shells'.
| A diagram of an atom. |
- In an atom the number of electrons is always the same as the number of protons in the nucleus.
- Different atoms can have different numbers of protons and neutrons.
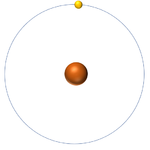
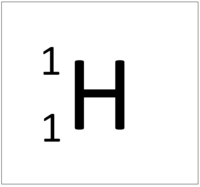
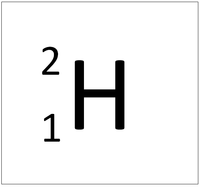
- The simplest atom is Hydrogen which has 1 proton and 1 electron and no neutrons.
Key Stage 4
Meaning
An atom is a very small particle made of protons, neutrons and electrons that can join with other atoms to make molecules.
About Atoms
- Atoms consist of a small, central nucleus containing protons and neutrons surrounded by electrons orbiting the nucleus.
- The electrons orbit the nucleus in so called 'electron shells.
| A diagram of an atom. |
- In an atom there is always the same number of protons as electrons. If any electron is added or removed the atom becomes an ion.
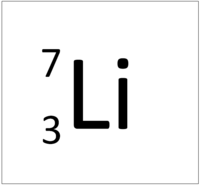
| Hydrogen | Helium | Lithium | Beryllium |
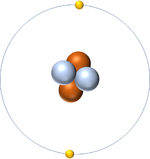
| Hydrogen always has 1 proton. | Helium always has 2 protons. | Lithium always has 3 protons. | Beryllium always has 4 protons. |
- Atoms of the same element can have different numbers of neutrons so they can be different isotopes of the same element.
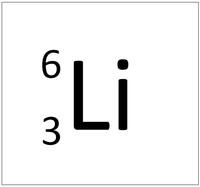
| Hydrogen-1 | Hydrogen-2 | Lithium-7 | Lithium-6 |
| Hydrogen always has 1 proton but in this case has no neutrons. | Hydrogen always has 1 proton but in this case also has a neutron. This isotope of Hydrogen is known as Deuterium. | Lithium always has 3 protons but in this case has 4 neutrons. | Lithium always has 3 protons but in this case has 3 neutrons. This particular isotope of Lithium is unstable and will radioactively decay. |
History of Atoms
- The existence and structure of atoms was not always known.