Key Stage 4
Meaning
Determining the specific heat capacity of a metal block.
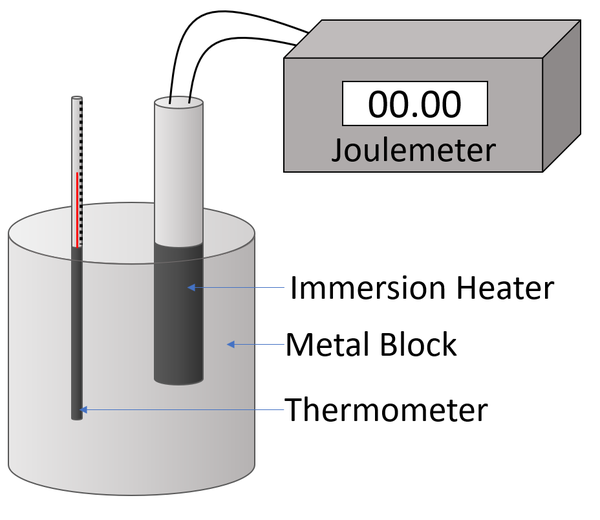
Experiment Version 1a: Joulemeter Changing the Temperature
Variables
- Independent Variable: The temperature of the metal block.
- Dependent Variable: The energy supplied to the metal block by heating.
- Control Variables: The mass of the metal block.
Method
- Measure the mass of the metal block using an electronic balance.
- Attach a Joulemeter and power supply to an immersion heater.
- Place the immersion heater and the thermometer in holes in the metal block.
- Place a drop of water in the thermometer hole to ensure thermal contact between the thermometer and the metal block.
- Read and record the initial temperature of the metal block.
- Switch on the power supply.
- Record the reading on the Joulemeter with every 2°C increase in temperature a minimum of 6 times.
- Plot a scatter graph with energy on the y-axis and temperature on the x-axis.
- Given the equation \(E_T=mc \Delta \theta\) then the gradient of the line of best fit will be the mass multiplied by the specific heat capacity (mc).
- Place the metal block on a heatproof mat to reduce the thermal energy lost to the table surface by conduction.
- Wrap the metal block a thermal insulator to reduce the thermal energy lost to the air.
- Complete the experiment in temperature range close to room temperature to reduce the rate of energy transfer from the metal block to the surroundings.
- Place the electronic balance on a flat, level surface to get an accurate reading of the mass.
- Use a data logger rather than a thermometer to reduce the random error caused by humans mistakes.
- Ensure the immersion heater and block begin at room temperature to reduce the error in repeat readings.
- Ensure the thickness and type of insulator is used for every repeat measurement reduce the error in repeat readings.
Experiment Version 1b: Joulemeter Changing the Energy
Variables
- Independent Variable: The energy supplied to the metal block by heating.
- Dependent Variable: The temperature of the metal block.
- Control Variables: The mass of the metal block.
Method
- Measure the mass of the metal block using an electronic balance.
- Attach a Joulemeter and power supply to an immersion heater.
- Place the immersion heater and the thermometer in holes in the metal block.
- Place a drop of water in the thermometer hole to ensure thermal contact between the thermometer and the metal block.
- Read and record the initial temperature of the metal block.
- Switch on the power supply.
- Record the reading on the thermometer with every 1000J shown on the joulemeter a minimum of 6 times.
- Plot a scatter graph with temperature on the y-axis and energy on the x-axis.
- Given the equation \(E_T=mc \Delta \theta\) then the gradient of the line of best fit will be \(\dfrac{1}{mc}\) where m = mass and c = specific heat capacity.
- Place the metal block on a heatproof mat to reduce the thermal energy lost to the table surface by conduction.
- Wrap the metal block a thermal insulator to reduce the thermal energy lost to the air.
- Complete the experiment in temperature range close to room temperature to reduce the rate of energy transfer from the metal block to the surroundings.
- Place the electronic balance on a flat, level surface to get an accurate reading of the mass.
- Use a data logger rather than a thermometer to reduce the random error caused by humans mistakes.
- Ensure the immersion heater and block begin at room temperature to reduce the error in repeat readings.
- Ensure the thickness and type of insulator is used for every repeat measurement reduce the error in repeat readings.
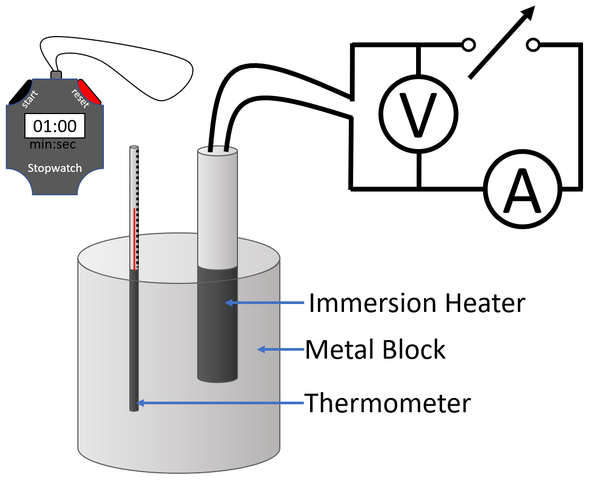
Experiment Version 2a: Ammeter, Voltmeter and Stopwatch Changing the Temperature
Variables
- Independent Variable: The temperature of the metal block.
- Dependent Variable: The time over which energy is supplied to the metal block.
- Control Variables: The mass of the metal block. The power of the immersion heater.
Method
- Measure the mass of the metal block using an electronic balance.
- Connect an Ammeter, power supply and immersion heater in series.
- Connect a voltmeter in parallel to the immersion heater.
- Place the immersion heater and the thermometer in holes in the metal block.
- Place a drop of water in the thermometer hole to ensure thermal contact between the thermometer and the metal block.
- Read and record the initial temperature of the metal block.
- Switch on the power supply, start a stopwatch and record the readings on the Voltmeter and Ammeter.#Record the time on the stopwatch with every 2°C increase in temperature a minimum of 6 times.
- Use the equation \(E = IVt\) to calculate the energy supplied to the metal block.
- Plot a scatter graph with energy on the y-axis and temperature on the x-axis.
- Given the equation \(E_T=mc \Delta \theta\) then the gradient of the line of best fit will be the mass multiplied by the specific heat capacity (mc).
- Place the metal block on a heatproof mat to reduce the thermal energy lost to the table surface by conduction.
- Wrap the metal block a thermal insulator to reduce the thermal energy lost to the air.
- Complete the experiment in temperature range close to room temperature to reduce the rate of energy transfer from the metal block to the surroundings.
- Place the electronic balance on a flat, level surface to get an accurate reading of the mass.
- Use a data logger rather than a thermometer to reduce the random error caused by humans mistakes.
- Ensure the immersion heater and block begin at room temperature to reduce the error in repeat readings.
- Ensure the thickness and type of insulator is used for every repeat measurement reduce the error in repeat readings.
Experiment Version 2b: Ammeter, Voltmeter and Stopwatch Changing the Time
Variables
- Independent Variable: The time over which energy is supplied to the metal block.
- Dependent Variable: The temperature of the metal block.
- Control Variables: The mass of the metal block. The power of the immersion heater.
Method
- Measure the mass of the metal block using an electronic balance.
- Connect an Ammeter, power supply and immersion heater in series.
- Connect a voltmeter in parallel to the immersion heater.
- Place the immersion heater and the thermometer in holes in the metal block.
- Place a drop of water in the thermometer hole to ensure thermal contact between the thermometer and the metal block.
- Read and record the initial temperature of the metal block.
- Switch on the power supply, start a stopwatch and record the readings on the Voltmeter and Ammeter.
- Read and record the temperature on the thermometer every 30 seconds on the stopwatch a minimum of 6 times.
- Use the equation \(E = IVt\) to calculate the energy supplied to the metal block.
- Plot a scatter graph with temperature on the y-axis and energy on the x-axis.
- Given the equation \(E_T=mc \Delta \theta\) then the gradient of the line of best fit will be \(\dfrac{1}{mc}\) where m = mass and c = specific heat capacity.
- Place the metal block on a heatproof mat to reduce the thermal energy lost to the table surface by conduction.
- Wrap the metal block a thermal insulator to reduce the thermal energy lost to the air.
- Complete the experiment in temperature range close to room temperature to reduce the rate of energy transfer from the metal block to the surroundings.
- Place the electronic balance on a flat, level surface to get an accurate reading of the mass.
- Use a data logger rather than a thermometer to reduce the random error caused by humans mistakes.
- Ensure the immersion heater and block begin at room temperature to reduce the error in repeat readings.
- Ensure the thickness and type of insulator is used for every repeat measurement reduce the error in repeat readings.