Contents
Key Stage 2
Meaning
Hydrogen is a gas that can catch fire easily.
Key Stage 3
Meaning
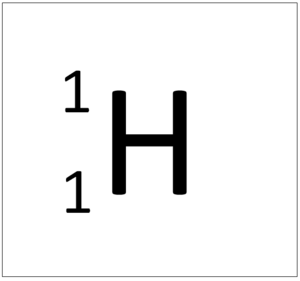
Hydrogen is a non-metal element with 1 proton in the nucleus.
About Hydrogen
Molecular Structure
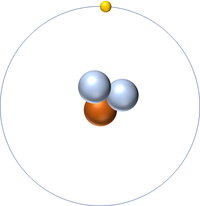
Atomic Structure
- Hydrogen has 1 proton and 0 neutrons so it has an atomic mass of 1.
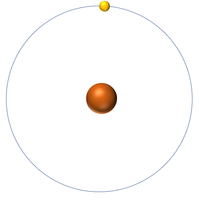
- An atom of hydrogen has one electron.
- A Hydrogen ion has usually lost its only electron to become positively charged.
Properties
- Hydrogen is a gas at room temperature.
- Hydrogen gas is less dense than air.
- Hydrogen reacts with Oxygen to form water.
Testing for Hydrogen
- Collect the gas in a test tube.
- Place a lit splint over the mouth of the test tube.
- If a 'squeaky pop' sound is made then the gas is Hydrogen.
Key Stage 4
Meaning
Hydrogen is a non-metal element with 1 proton in the nucleus.
About Hydrogen
Molecular Structure
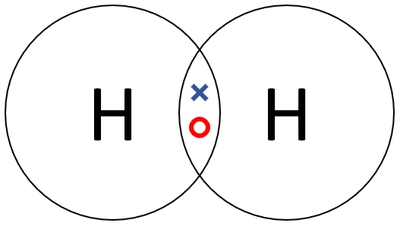
- Hydrogen has the chemical formula H2.
- Two Hydrogen atoms join together in a covalent bond.
| A dot and cross diagram of a Hydrogen molecule. |
Atomic Structure
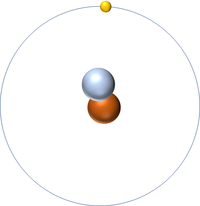
- The most common isotope of Hydrogen has 1 proton and 0 neutrons so it has an atomic mass of 1.
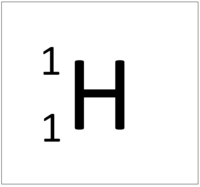
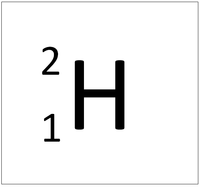
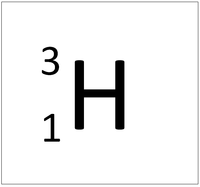
| Hydrogen | Deuterium | Tritium |
| Hydrogen always has 1 proton but isotope there are no neutrons. | Hydrogen always has 1 proton but in this isotope there is 1 neutron. This isotope of Hydrogen is known as Deuterium. | Hydrogen always has 1 proton but in this isotope there is 2 neutrons. This isotope of Hydrogen is known as Tritium. |
- An atom of hydrogen has one electron.
- A Hydrogen ion has usually lost its only electron to become positively charged.
Properties
- Hydrogen is a gas at standard temperature and pressure.
- Hydrogen gas is less dense than air.
- Hydrogen reacts with Oxygen to form water.