Difference between revisions of "Non-metal"
(→About Metals) |
(→Properties of Metals) |
||
| (6 intermediate revisions by 2 users not shown) | |||
| Line 13: | Line 13: | ||
===Properties of Metals=== | ===Properties of Metals=== | ||
| − | There are several key properties of [[metal]]s you should know. | + | There are several key properties of [[metal]]s you should know. '''Non-metals''' are: |
*Bad [[Electrical Conductor|conductor]]s of [[electricity]] | *Bad [[Electrical Conductor|conductor]]s of [[electricity]] | ||
*Bad [[Thermal Conductor|conductor]]s of [[thermal energy]] | *Bad [[Thermal Conductor|conductor]]s of [[thermal energy]] | ||
| Line 20: | Line 20: | ||
*[[Ductility|Stiff]] - They do not stretch easily. | *[[Ductility|Stiff]] - They do not stretch easily. | ||
*[[Sonorousness|Not sonorous]] - They make a short dull [[sound]] when hit. | *[[Sonorousness|Not sonorous]] - They make a short dull [[sound]] when hit. | ||
| + | |||
| + | ==Key Stage 4== | ||
| + | ===Meaning=== | ||
| + | A [[Non-metal]] is a a [[material]] that is a bad [[Electrical Conductor|conductor]] of [[electricity]] and a bad [[Thermal Conductor|conductor]] of [[thermal energy]]. | ||
| + | |||
| + | ===About Metals=== | ||
| + | : [[Non-metal]]s are found on the right hand side of the [[Periodic Table]] | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:PeriodicTableKS4.png|center|800px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |This [[Periodic Table]] shows the [[Non-metal]] [[element]]s on the right in orange, green and grey. | ||
| + | |} | ||
| + | : [[Non-metal]]s are on the right hand side of the [[Periodic Table]] because most of those [[element]]s gain [[electron]]s to form [[Negative Ion|negative ions]] in [[compound]]s with [[metal]]s. | ||
| + | : [[Non-metal]] [[element]]s form [[Covalent Bond|covalent bonds]] with other [[non-metal]] [[element]]s. | ||
| + | : [[Non-metal]] [[element]]s usually make poor [[Electrical Conductor|electrical]] and [[Thermal Conductor|thermal]] [[conductor]]s [[electron]]s are shared but not easily transferred between [[adjacent]] [[atom]]s. | ||
| + | : [[Non-metal]]s [[element]]s have a range of [[Melting Point|melting]] and [[Boiling Point|boiling]] points with some which are [[solid]] at [[Room Temperature|room temperature]] and others [[gas]]eous and one [[liquid]] ([[Bromine]]). | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Non-metal, properties, pages 34-5, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945598/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945598&linkCode=as2&tag=nrjc-21&linkId=ad276ad49df77ab4b40ab4fd0fe10110 ''Non-metals, page 108, GCSE Combined Science; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851354/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851354&linkCode=as2&tag=nrjc-21&linkId=9012a0d354024419214fb3ad5ac44ba0 ''Non-metals, page 125, GCSE Combined Science Trilogy 1, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945571/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945571&linkCode=as2&tag=nrjc-21&linkId=9e29fad914244909903e5e93f8a01d194 ''Non-metals, pages 23, 28, GCSE Chemistry; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Non-metals, pages 24-25, 27-29, 31, 40-41, 45, 48-51, 86, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294639X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294639X&linkCode=as2&tag=nrjc-21&linkId=51599bb45a2bfaf7c1b6a978b2ca2616 ''Non-metals, pages 54, 55, 70, GCSE Combined Science Trilogy; Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Non-metals, pages 54, 55, 72, GCSE Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Non-metals; properties of, page 10, GCSE Chemistry, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851354/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851354&linkCode=as2&tag=nrjc-21&linkId=9012a0d354024419214fb3ad5ac44ba0 ''Non-metals; reaction with metals, pages 152-3, 249, GCSE Combined Science Trilogy 1, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Non-metals; reaction with metals, pages 37-8, GCSE Chemistry, Hodder, AQA ''] | ||
| + | |||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1292120193/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120193&linkCode=as2&tag=nrjc-21&linkId=572df39392fb4200db8391d98ae6314e ''Non-metals, page 190, GCSE Combined Science, Pearson Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945725/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945725&linkCode=as2&tag=nrjc-21&linkId=694be7494de75af3349537d34e13f7f0 ''Non-metals, page 25, GCSE Chemistry; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Non-metals, page 46, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945741/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945741&linkCode=as2&tag=nrjc-21&linkId=30da4f2178da182547b62a7329d13b57 ''Non-metals, page 88, GCSE Combined Science; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948147/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948147&linkCode=as2&tag=nrjc-21&linkId=f63dcd8345f4e49c717b39a228a36c7c ''Non-metals, pages 41, 66, GCSE Chemistry, CGP, Edexcel ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945679/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945679&linkCode=as2&tag=nrjc-21&linkId=a2db42f7b4bdf10cafaafa3bb9120940 ''Non-metals, page 24, Gateway GCSE Chemistry; The Revision Guide, CGP, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945695/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945695&linkCode=as2&tag=nrjc-21&linkId=ceafcc80bcad6b6754ee97a0c7ceea53 ''Non-metals, page 96, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR ''] | ||
Latest revision as of 22:16, 26 February 2022
Contents
Key Stage 3
Meaning
A Non-metal is a a material that is a bad conductor of electricity and a bad conductor of thermal energy.
About Metals
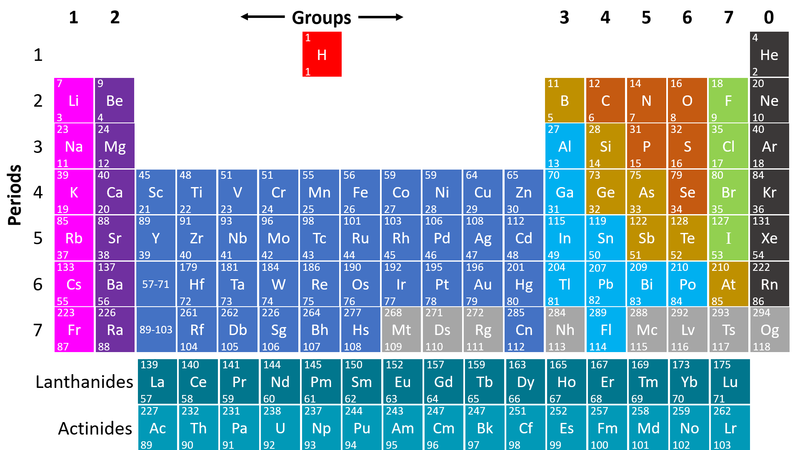
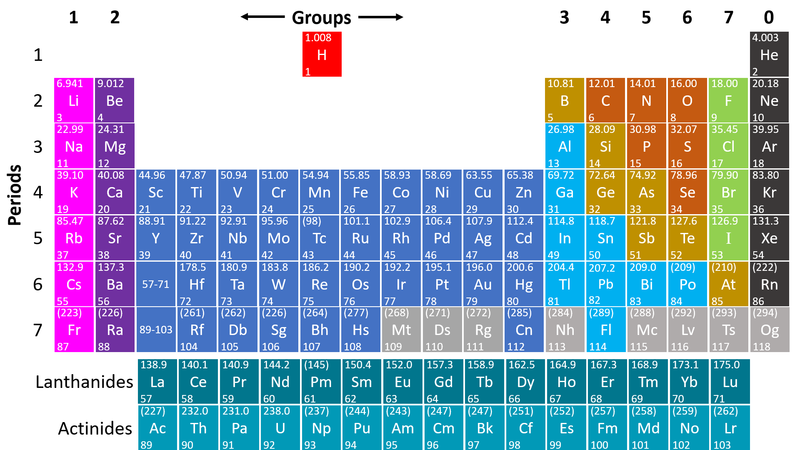
- Non-metals are found on the right hand side of the Periodic Table
| This Periodic Table shows the Non-metal elements on the right in orange, green and grey. |
Properties of Metals
There are several key properties of metals you should know. Non-metals are:
- Bad conductors of electricity
- Bad conductors of thermal energy
- Dull - They do not have reflective surfaces.
- Brittle - They break easily when hit.
- Stiff - They do not stretch easily.
- Not sonorous - They make a short dull sound when hit.
Key Stage 4
Meaning
A Non-metal is a a material that is a bad conductor of electricity and a bad conductor of thermal energy.
About Metals
- Non-metals are found on the right hand side of the Periodic Table
| This Periodic Table shows the Non-metal elements on the right in orange, green and grey. |
- Non-metals are on the right hand side of the Periodic Table because most of those elements gain electrons to form negative ions in compounds with metals.
- Non-metal elements form covalent bonds with other non-metal elements.
- Non-metal elements usually make poor electrical and thermal conductors electrons are shared but not easily transferred between adjacent atoms.
- Non-metals elements have a range of melting and boiling points with some which are solid at room temperature and others gaseous and one liquid (Bromine).
References
AQA
- Non-metal, properties, pages 34-5, GCSE Chemistry; Student Book, Collins, AQA
- Non-metals, page 108, GCSE Combined Science; The Revision Guide, CGP, AQA
- Non-metals, page 125, GCSE Combined Science Trilogy 1, Hodder, AQA
- Non-metals, pages 23, 28, GCSE Chemistry; The Revision Guide, CGP, AQA
- Non-metals, pages 24-25, 27-29, 31, 40-41, 45, 48-51, 86, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Non-metals, pages 54, 55, 70, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Non-metals, pages 54, 55, 72, GCSE Chemistry, CGP, AQA
- Non-metals; properties of, page 10, GCSE Chemistry, Hodder, AQA
- Non-metals; reaction with metals, pages 152-3, 249, GCSE Combined Science Trilogy 1, Hodder, AQA
- Non-metals; reaction with metals, pages 37-8, GCSE Chemistry, Hodder, AQA
Edexcel
- Non-metals, page 190, GCSE Combined Science, Pearson Edexcel
- Non-metals, page 25, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Non-metals, page 46, GCSE Chemistry, Pearson, Edexcel
- Non-metals, page 88, GCSE Combined Science; The Revision Guide, CGP, Edexcel
- Non-metals, pages 41, 66, GCSE Chemistry, CGP, Edexcel