Difference between revisions of "Non-metal"
| Line 33: | Line 33: | ||
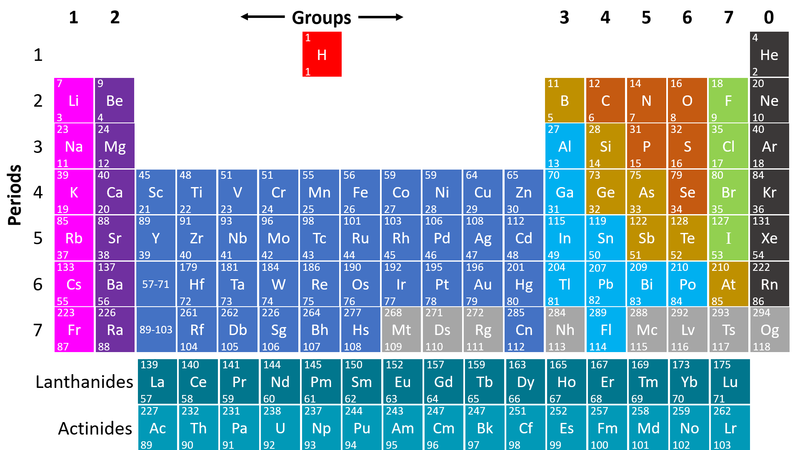
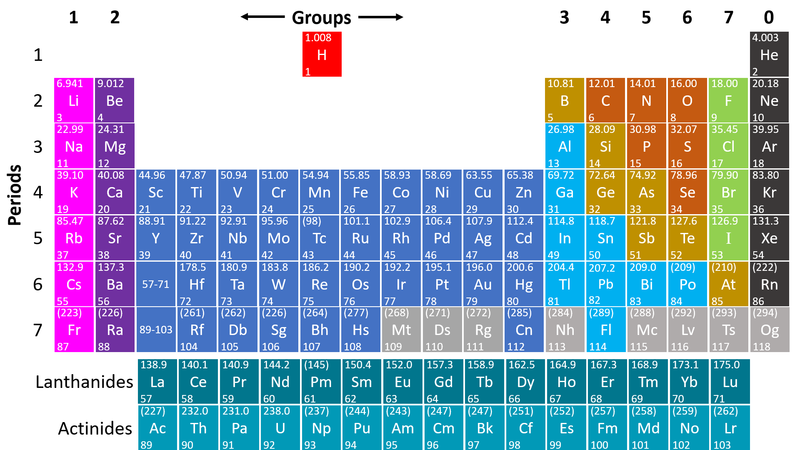
| style="height:20px; width:200px; text-align:center;" |This [[Periodic Table]] shows the [[Non-metal]] [[element]]s on the right in orange, green and grey. | | style="height:20px; width:200px; text-align:center;" |This [[Periodic Table]] shows the [[Non-metal]] [[element]]s on the right in orange, green and grey. | ||
|} | |} | ||
| − | : | + | : [[Non-metal]]s are on the right hand side of the [[Periodic Table]] because most of those [[element]]s gain [[electron]]s to form [[Negative Ion|negative ions]] in [[compound]]s with [[metal]]s. |
: [[Non-metal]] [[element]]s form [[Covalent Bond|covalent bonds]] with other [[non-metal]] [[element]]s. | : [[Non-metal]] [[element]]s form [[Covalent Bond|covalent bonds]] with other [[non-metal]] [[element]]s. | ||
: [[Non-metal]] [[element]]s usually make poor [[Electrical Conductor|electrical]] and [[Thermal Conductor|thermal]] [[conductor]]s [[electron]]s are shared but not easily transferred between [[adjacent]] [[atom]]s. | : [[Non-metal]] [[element]]s usually make poor [[Electrical Conductor|electrical]] and [[Thermal Conductor|thermal]] [[conductor]]s [[electron]]s are shared but not easily transferred between [[adjacent]] [[atom]]s. | ||
: [[Non-metal]]s [[element]]s have a range of [[Melting Point|melting]] and [[Boiling Point|boiling]] points with some which are [[solid]] at [[Room Temperature|room temperature]] and others [[gas]]eous and one [[liquid]] ([[Bromine]]). | : [[Non-metal]]s [[element]]s have a range of [[Melting Point|melting]] and [[Boiling Point|boiling]] points with some which are [[solid]] at [[Room Temperature|room temperature]] and others [[gas]]eous and one [[liquid]] ([[Bromine]]). | ||
Revision as of 11:22, 9 December 2018
Contents
Key Stage 3
Meaning
A Non-metal is a a material that is a bad conductor of electricity and a bad conductor of thermal energy.
About Metals
- Non-metals are found on the right hand side of the Periodic Table
| This Periodic Table shows the Non-metal elements on the right in orange, green and grey. |
Properties of Metals
There are several key properties of metals you should know. Metals are:
- Bad conductors of electricity
- Bad conductors of thermal energy
- Dull - They do not have reflective surfaces.
- Brittle - They break easily when hit.
- Stiff - They do not stretch easily.
- Not sonorous - They make a short dull sound when hit.
Key Stage 3
Meaning
A Non-metal is a a material that is a bad conductor of electricity and a bad conductor of thermal energy.
About Metals
- Non-metals are found on the right hand side of the Periodic Table
| This Periodic Table shows the Non-metal elements on the right in orange, green and grey. |
- Non-metals are on the right hand side of the Periodic Table because most of those elements gain electrons to form negative ions in compounds with metals.
- Non-metal elements form covalent bonds with other non-metal elements.
- Non-metal elements usually make poor electrical and thermal conductors electrons are shared but not easily transferred between adjacent atoms.
- Non-metals elements have a range of melting and boiling points with some which are solid at room temperature and others gaseous and one liquid (Bromine).