Difference between revisions of "Period (Chemistry)"
| Line 43: | Line 43: | ||
The [[reactivity]] decreases as you go across the [[Period (Chemistry)|period]] because: | The [[reactivity]] decreases as you go across the [[Period (Chemistry)|period]] because: | ||
*The outer [[electron]]s are all roughly the same distance away from the [[Atomic Nucleus|nucleus]]. | *The outer [[electron]]s are all roughly the same distance away from the [[Atomic Nucleus|nucleus]]. | ||
| − | *The [[charge]] on the [[Atomic Nucleus|atomic nucleus]] increases as you move go across the [[period]] but the [[electron]] shielding caused by the two inner [[electron]]s remains the same. This causes the [[electron]]s to experience a greater [[force]] of [[attraction]] as you move along the [[period]], making it harder for the [[atom]]s to lose [[electron]]s and become [[ion]]s. | + | *The [[Electrical Charge|charge]] on the [[Atomic Nucleus|atomic nucleus]] increases as you move go across the [[period]] but the [[electron]] shielding caused by the two inner [[electron]]s remains the same. This causes the [[electron]]s to experience a greater [[force]] of [[attraction]] as you move along the [[period]], making it harder for the [[atom]]s to lose [[electron]]s and become [[ion]]s. |
[[Nitrogen]], [[Oxygen]] and [[Fluorine]] can all gain [[electron]]s to become [[Negative Charge|negative]] [[ion]]s in certain [[Chemical Reaction|reaction]]s. | [[Nitrogen]], [[Oxygen]] and [[Fluorine]] can all gain [[electron]]s to become [[Negative Charge|negative]] [[ion]]s in certain [[Chemical Reaction|reaction]]s. | ||
| Line 49: | Line 49: | ||
The [[reactivity]] increases as you go across the [[Period (Chemistry)|period]] because: | The [[reactivity]] increases as you go across the [[Period (Chemistry)|period]] because: | ||
*The outer [[electron]]s are all roughly the same distance away from the [[Atomic Nucleus|nucleus]]. | *The outer [[electron]]s are all roughly the same distance away from the [[Atomic Nucleus|nucleus]]. | ||
| − | *The [[charge]] on the [[Atomic Nucleus|atomic nucleus]] increases as you move go across the [[period]] but the [[electron]] shielding caused by the two inner [[electron]]s remains the same. This causes the [[electron]]s to experience a greater [[force]] of [[attraction]] as you move along the [[period]], making it easier for an [[atom]]s to gain more [[electron]]s to become [[ion]]s. | + | *The [[Electrical Charge|charge]] on the [[Atomic Nucleus|atomic nucleus]] increases as you move go across the [[period]] but the [[electron]] shielding caused by the two inner [[electron]]s remains the same. This causes the [[electron]]s to experience a greater [[force]] of [[attraction]] as you move along the [[period]], making it easier for an [[atom]]s to gain more [[electron]]s to become [[ion]]s. |
|} | |} | ||
Latest revision as of 10:54, 2 February 2019
Contents
Key Stage 3
Meaning
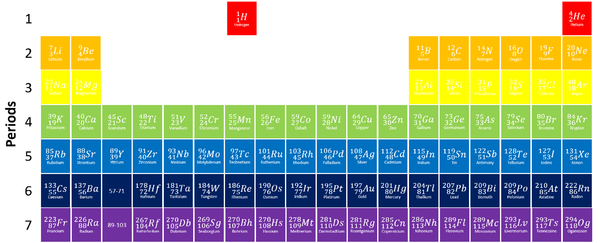
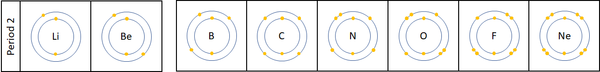
A period is a row on the Periodic Table whose elements all have the same number of Electron Shells.
About Periods
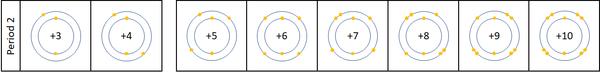
- Atomic Number increases as you move along a period.
Trends within Periods
- The chemical and physical properties of elements change as you move along a period.
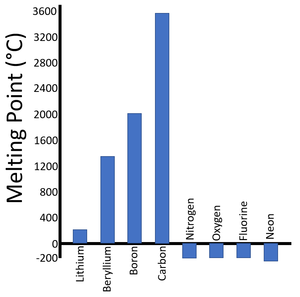
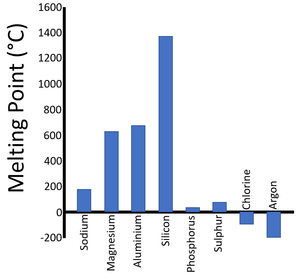
| Period 2 Melting Points | Period 3 Melting Points |
| There is a trend in the Melting Points as you move along the period. | A similar trend can be seen in the next period. |
Key Stage 4
Meaning
A period is a row on the Periodic Table whose elements all have the same number of Electron Shells.
About Periods
- Atomic Number increases as you move along a period.
- The number of the period is the same as the number of electron shells.
Trends within Periods
| For the first 3 elements Lithium, Beryllium and Boron all lose electrons in chemical reactions.
The reactivity decreases as you go across the period because:
Nitrogen, Oxygen and Fluorine can all gain electrons to become negative ions in certain reactions. The reactivity increases as you go across the period because:
|