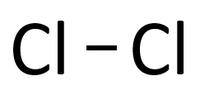Difference between revisions of "Single Bond"
(→Examples) |
(→Examples) |
||
| Line 14: | Line 14: | ||
|[[File:BallandStickWater.png|center|200px]] | |[[File:BallandStickWater.png|center|200px]] | ||
|- | |- | ||
| − | | style="height:20px; width:200px; text-align:center;" | | + | | style="height:20px; width:200px; text-align:center;" |In this [[Structural Diagram|structural diagram]] a '''single bond''' is shown between two [[Chlorine]] [[atom]]s. |
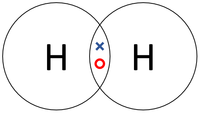
| − | | style="height:20px; width:200px; text-align:center;" | | + | | style="height:20px; width:200px; text-align:center;" |In this [[Dot and Cross Diagram|dot and cross diagram]] the two [[Hydrogen]] [[atom]]s in a [[Hydrogen]] [[molecule]] are shown to each share only one [[electron]] in a '''single bond'''. |
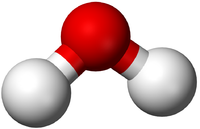
| − | | style="height:20px; width:200px; text-align:center;" | | + | | style="height:20px; width:200px; text-align:center;" |In this [[Ball and Stick Model|ball and stick model]] of [[Water]] the [[Oxygen]] in [[Water]] shares one [[electron]] (shown by one stick) with each [[Hydrogen]] [[atom]] forming two '''single bonds'''. |
|} | |} | ||
Latest revision as of 13:19, 14 January 2019
Key Stage 4
Meaning
A single bond is a chemical bond in which only one electron is shared or transferred from the outer shell.
About Single Bonds
- In covalent bonds a single bond means one electron from the outer shell of an atom is shared.
- In ionic bonds a single bond means one of the atoms has gained or lost just one electron that has been transferred to another element.
Examples
| In this structural diagram a single bond is shown between two Chlorine atoms. | In this dot and cross diagram the two Hydrogen atoms in a Hydrogen molecule are shown to each share only one electron in a single bond. | In this ball and stick model of Water the Oxygen in Water shares one electron (shown by one stick) with each Hydrogen atom forming two single bonds. |