Difference between revisions of "Chemical Reaction"
| (25 intermediate revisions by 2 users not shown) | |||
| Line 10: | Line 10: | ||
*[[Combustion|Burning]] | *[[Combustion|Burning]] | ||
*[[Vinegar and Bicarbonate of Soda|Adding Bicarbonate of Soda to Vinegar]]. | *[[Vinegar and Bicarbonate of Soda|Adding Bicarbonate of Soda to Vinegar]]. | ||
| + | |||
| + | ==Key Stage 3== | ||
| + | ===Meaning=== | ||
| + | A [[Chemical Reaction]] is when the [[atom]]s in [[molecule]]s break their [[Chemical Bond|bonds]] and form new [[Chemical Bond|bonds]], usually with other [[atom]]s. | ||
| + | |||
| + | ===About Chemical Reactions=== | ||
| + | : Most '''chemical reactions''' are irreversible. | ||
| + | : In a '''chemical reaction''' the [[atom]]s rearrange to form a new [[substance]]. | ||
| + | : '''Chemical reactions''' often need some [[energy]] to start, like [[combustion]] where the [[fuel]] needs to be [[Heat|heated]] to start. | ||
| + | : If a '''chemical reaction''' gives off more energy than it needs to start then it is called an [[exothermic]] '''reaction'''. In these '''reactions''' the [[temperature]] increases. | ||
| + | : If a '''chemical reaction''' takes in more energy than it gives out then it is called an [[endothermic]] '''reaction'''. In these '''reactions''' the [[temperature]] decreases. | ||
| + | |||
| + | ===Signs of Chemical Reactions=== | ||
| + | There are three signs that a '''chemical reaction''' is taking place: | ||
| + | *A colour change. | ||
| + | *A temperature change. | ||
| + | *[[Effervescence]] (bubbles of [[gas]] are given off. | ||
| + | |||
| + | ===Examples=== | ||
| + | |||
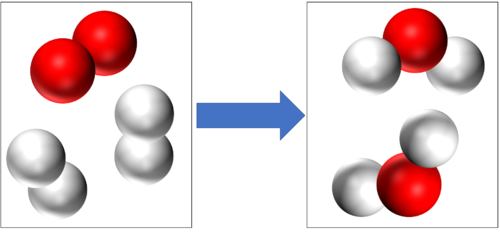
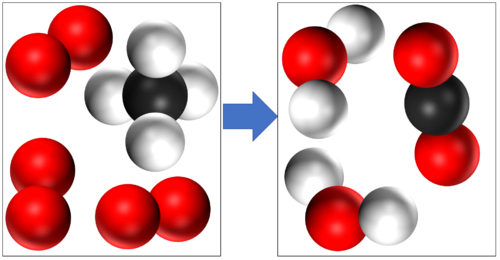
| + | These are two examples of [[Combustion]] | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:RearrangeAtoms1.png|center|500px]] | ||
| + | |- | ||
| + | | style="height:20px; width:500px; text-align:center;" | | ||
| + | [[Hydrogen]] + [[Oxygen]] → [[Water]] | ||
| + | |||
| + | 2H<sub>2</sub> + O<sub>2</sub> → 2H<sub>2</sub>O | ||
| + | |} | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:RearrangeAtoms2.png|center|500px]] | ||
| + | |- | ||
| + | | style="height:20px; width:500px; text-align:center;" | | ||
| + | [[Methane]] + [[Oxygen]] → [[Water]] + [[Carbon Dioxide]] | ||
| + | |||
| + | CH<sub>4</sub> + 2O<sub>2</sub> → 2H<sub>2</sub>O + CO<sub>2</sub> | ||
| + | |} | ||
| + | |||
| + | ===Types of Chemical Reaction=== | ||
| + | *[[Combustion]] - A [[chemical]] burns in the presence of [[Oxygen]]. | ||
| + | *[[Thermal Decomposition]] - A [[compound]] breaks down into smaller [[compound]]s. | ||
| + | *[[Oxidation]] - A [[chemical]] '''reacts''' with [[Oxygen]]. | ||
| + | *[[Displacement Reaction]] - One [[element]] replaces another in a [[compound]]. | ||
| + | *[[Neutralisation]] - An [[acid]] and a [[base]] '''react''' to make a [[salt]]. | ||
| + | |||
| + | ==Key Stage 4== | ||
| + | ===Meaning=== | ||
| + | A [[Chemical Reaction]] is when the [[atom]]s in [[molecule]]s break their [[Chemical Bond|bonds]] and form new [[Chemical Bond|bonds]], usually with other [[atom]]s. | ||
| + | |||
| + | ===About Chemical Reactions=== | ||
| + | : In a '''chemical reaction''' the [[atom]]s rearrange to form a new [[substance]]. | ||
| + | : '''Chemical reactions''' often need some [[energy]] to start, like [[combustion]] where the [[fuel]] needs to be [[Heat|heated]] to start. | ||
| + | : If a '''chemical reaction''' gives off more energy than it needs to start then it is called an [[exothermic]] '''reaction'''. In these '''reactions''' the [[temperature]] increases. | ||
| + | : If a '''chemical reaction''' takes in more energy than it gives out then it is called an [[endothermic]] '''reaction'''. In these '''reactions''' the [[temperature]] decreases. | ||
| + | There are three ways that '''chemical reactions''' can happen: | ||
| + | *[[Electron Transfer]] - [[Electron]]s from the [[Outer Shell]] of one [[atom]] move to the [[Outer Shell]] of another [[atom]] to form an [[Ionic Bond]]. | ||
| + | *[[Proton Transfer]] - [[Hydrogen#Ion|Hydrogen Ions]] are removed from an [[acid]] and added to a [[base]]. | ||
| + | *[[Electron Sharing]] - [[Electron]]s in the [[Outer Shell]] of one [[atom]] are shared with the [[Outer Shell]] of another [[atom]] forming a [[Covalent Bond|covalent bond]]. | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/0008158770/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158770&linkCode=as2&tag=nrjc-21&linkId=ec31595e720e1529e49876c3866fff6e ''Chemical reaction, page 108, GCSE Physics; Student Book, Collins, AQA ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359837/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359837&linkCode=as2&tag=nrjc-21&linkId=3c4229e8b023b2b60768e7ea2307cc6f ''Chemical reactions, pages 27, Gateway GCSE Physics, Oxford, OCR ''] | ||
| + | |||
| + | ==Beyond the Curriculum== | ||
| + | {{#ev:youtube|https://www.youtube.com/watch?v=8m6RtOpqvtU}} | ||
Latest revision as of 13:36, 1 December 2019
Contents
Key Stage 2
Meaning
A chemical reaction is when one substance changes into another.
About Chemical Reactions
- Most chemical reactions are irreversible.
- Chemical reactions usually cause a change in temperature.
- Chemical reactions you should know are:
Key Stage 3
Meaning
A Chemical Reaction is when the atoms in molecules break their bonds and form new bonds, usually with other atoms.
About Chemical Reactions
- Most chemical reactions are irreversible.
- In a chemical reaction the atoms rearrange to form a new substance.
- Chemical reactions often need some energy to start, like combustion where the fuel needs to be heated to start.
- If a chemical reaction gives off more energy than it needs to start then it is called an exothermic reaction. In these reactions the temperature increases.
- If a chemical reaction takes in more energy than it gives out then it is called an endothermic reaction. In these reactions the temperature decreases.
Signs of Chemical Reactions
There are three signs that a chemical reaction is taking place:
- A colour change.
- A temperature change.
- Effervescence (bubbles of gas are given off.
Examples
These are two examples of Combustion
|
2H2 + O2 → 2H2O |
|
Methane + Oxygen → Water + Carbon Dioxide CH4 + 2O2 → 2H2O + CO2 |
Types of Chemical Reaction
- Combustion - A chemical burns in the presence of Oxygen.
- Thermal Decomposition - A compound breaks down into smaller compounds.
- Oxidation - A chemical reacts with Oxygen.
- Displacement Reaction - One element replaces another in a compound.
- Neutralisation - An acid and a base react to make a salt.
Key Stage 4
Meaning
A Chemical Reaction is when the atoms in molecules break their bonds and form new bonds, usually with other atoms.
About Chemical Reactions
- In a chemical reaction the atoms rearrange to form a new substance.
- Chemical reactions often need some energy to start, like combustion where the fuel needs to be heated to start.
- If a chemical reaction gives off more energy than it needs to start then it is called an exothermic reaction. In these reactions the temperature increases.
- If a chemical reaction takes in more energy than it gives out then it is called an endothermic reaction. In these reactions the temperature decreases.
There are three ways that chemical reactions can happen:
- Electron Transfer - Electrons from the Outer Shell of one atom move to the Outer Shell of another atom to form an Ionic Bond.
- Proton Transfer - Hydrogen Ions are removed from an acid and added to a base.
- Electron Sharing - Electrons in the Outer Shell of one atom are shared with the Outer Shell of another atom forming a covalent bond.