Difference between revisions of "Electrolysis"
(→Key Stage 4) |
|||
| Line 16: | Line 16: | ||
: When the [[Negative Ion|negative ions]] reach the [[anode]] they lose [[electron]]s to become [[atom]]s or [[Neutral (Charge)|neutral]] [[compound]]s. | : When the [[Negative Ion|negative ions]] reach the [[anode]] they lose [[electron]]s to become [[atom]]s or [[Neutral (Charge)|neutral]] [[compound]]s. | ||
: When the [[Positive Ion|positive ions]] reach the [[cathode]] they gain [[electron]]s to become [[atom]]s. However, in [[aqueous]] [[solution]] if the [[metal]] [[ion]]s are more [[reactivity|reactive]] than [[Hydrogen]] then [[Hydrogen]] [[gas]] will be [[product|produced]]. | : When the [[Positive Ion|positive ions]] reach the [[cathode]] they gain [[electron]]s to become [[atom]]s. However, in [[aqueous]] [[solution]] if the [[metal]] [[ion]]s are more [[reactivity|reactive]] than [[Hydrogen]] then [[Hydrogen]] [[gas]] will be [[product|produced]]. | ||
| + | : To describe an [[electrolysis]] [[Chemical Reaction|reaction]] [[Half Equation|half equations]] are used. | ||
| + | |||
| + | ===Examples=== | ||
| + | [[Balanced Symbol Equation]]: 2Li<sub>2</sub>O → 4Li + O<sub>2</sub> | ||
| + | |||
| + | '''Half Equation''' at [[cathode]]: Li<sup>+</sup> + e<sup>-</sup> → Li | ||
| + | |||
| + | '''Half Equation''' at [[anode]]: 2O<sup>-2</sup> → O<sub>2</sub> + 4e<sup>-</sup> | ||
| + | |||
| + | {| class="wikitable" | ||
| + | | style="height:10px; width:200px; text-align:center;" |[[Balanced Symbol Equation]] | ||
| + | | style="height:10px; width:200px; text-align:center;" |'''2Li<sub>2</sub>O(l) → 4Li(l) + O<sub>2</sub>(g)''' | ||
| + | | style="height:10px; width:200px; text-align:center;" |'''CuCl<sub>2</sub>(aq) → Cu(s) + Cl<sub>2</sub>(g)''' | ||
| + | | style="height:10px; width:200px; text-align:center;" |'''2H<sub>2</sub>O(l) → 2H<sub>2</sub>(g) + O<sub>2</sub>(g)''' | ||
| + | |- | ||
| + | | style="height:10px; width:200px; text-align:center;" |'''Half Equation''' at [[cathode]] | ||
| + | | style="height:10px; width:200px; text-align:center;" |Li<sup>+</sup> + e<sup>-</sup> → Li | ||
| + | | style="height:10px; width:200px; text-align:center;" |Cu<sup>+2</sup>(aq) + 2e<sup>-</sup> → Cu(s) | ||
| + | | style="height:10px; width:200px; text-align:center;" |2H<sup>+</sup>(aq) + 2e<sup>-</sup> → H<sub>2</sub>(g) | ||
| + | |- | ||
| + | | style="height:10px; width:200px; text-align:center;" |'''Half Equation''' at [[anode]] | ||
| + | | style="height:10px; width:200px; text-align:center;" |2O<sup>-2</sup> → O<sub>2</sub> + 4e<sup>-</sup> | ||
| + | | style="height:10px; width:200px; text-align:center;" |2Cl<sup>-</sup>(aq) → Cl<sub>2</sub>(g) + 2e<sup>-</sup> | ||
| + | | style="height:10px; width:200px; text-align:center;" |4OH<sup>-</sup> → 2H<sub>2</sub>O(l) + O<sub>2</sub>(g) + 4e<sup>-</sup> | ||
| + | |} | ||
Revision as of 20:15, 13 January 2019
Contents
Key Stage 3
Meaning
Electrolysis is a process where compounds are decomposed by an electrical current.
About Electrolysis
- Electrolysis can be used to extract metals from minerals when the metal is more reactive than Carbon.
Key Stage 4
Meaning
Electrolysis is a process in which an ionic compound is decomposed by passing an electrical current through it.
About Electrolysis
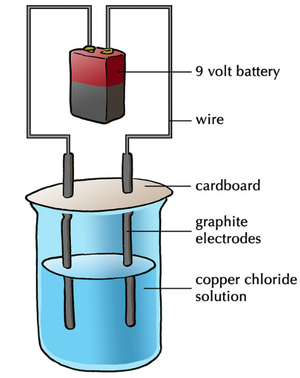
- In electrolysis two electrodes (an anode and a cathode) are placed in either a molten or aqueous ionic compound.
- The positive ions are attracted to the negative electrode (cathode) and the negative ions are attracted to the positive electrode (anode).
- When the negative ions reach the anode they lose electrons to become atoms or neutral compounds.
- When the positive ions reach the cathode they gain electrons to become atoms. However, in aqueous solution if the metal ions are more reactive than Hydrogen then Hydrogen gas will be produced.
- To describe an electrolysis reaction half equations are used.
Examples
Balanced Symbol Equation: 2Li2O → 4Li + O2
Half Equation at cathode: Li+ + e- → Li
Half Equation at anode: 2O-2 → O2 + 4e-
| Balanced Symbol Equation | 2Li2O(l) → 4Li(l) + O2(g) | CuCl2(aq) → Cu(s) + Cl2(g) | 2H2O(l) → 2H2(g) + O2(g) |
| Half Equation at cathode | Li+ + e- → Li | Cu+2(aq) + 2e- → Cu(s) | 2H+(aq) + 2e- → H2(g) |
| Half Equation at anode | 2O-2 → O2 + 4e- | 2Cl-(aq) → Cl2(g) + 2e- | 4OH- → 2H2O(l) + O2(g) + 4e- |