Atomic Structure
Key Stage 4
Meaning
Atomic structure is what an atom is made of and how those parts are arranged.
About the Atomic Structure
- The atomic structure was not always known and has gone through several advances; The Dalton Model the Plumb Pudding Model the Nuclear Model and the Bohr Model.
- Atoms are now known to be made of three smaller particles; the proton, neutron and electron.
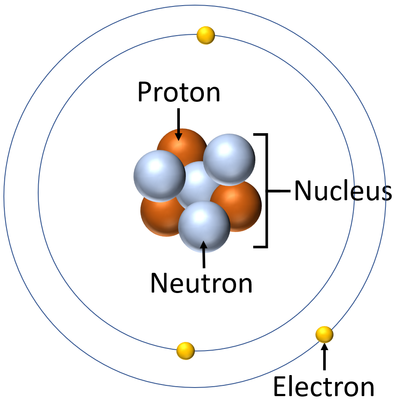
- Protons and neutrons are found in the nucleus at the centre of an atom. Electrons are found orbiting the nucleus in 'shells'.
| A diagram of an atom showing the subatomic particles. |
The Subatomic Particles
- The protons and neutrons are known as nucleons because they are found in the nucleus. The electrons are not part of the nucleus so they are not nucleons.
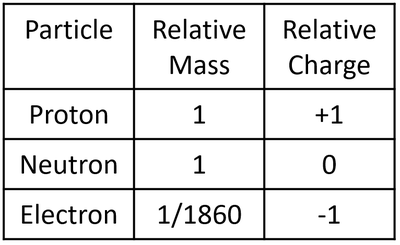
- These particles all have slightly different properties. Since the subatomic particles are so small their mass and charge are extremely small numbers so, to make it easier, they are represented as 'relative' mass and 'relative' charge compared to a proton.
| A table showing the relative mass and relative charge of the proton, neutron and electron. |