Contents
Key Stage 4
Meaning
A chemical bond is a force of attraction holding the atoms inside a molecule together.
About Chemical Bonds
There are three types of chemical bond you should know:
- Covalent Bonds - In which atoms share electrons with one another.
- Ionic Bonds - In which electrons are transferred from one atom to another.
- Metallic Bonds - In which some electrons move freely between atoms creating lattice of positively charged ions surrounded by a sea of delocalised electrons (free electrons).
Examples
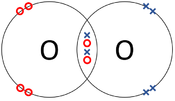
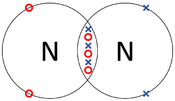
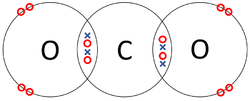
| The two Oxygen atoms each share two of their electrons with one another. | The two Nitrogen atoms each share three of their electrons with one another. | Each Oxygen shares two of its electrons with the Carbon atom while the Carbon atom shares two electrons with each Oxygen atom. |
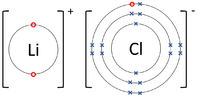
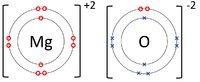
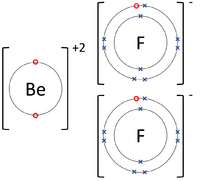
| The Lithium atom donates an electron from its outer shell to the outer shell of the Fluorine atom. | The Magnesium atom donates two electrons from its outer shell to the outer shell of the Oxygen atom. | The Beryllium atom donates two electrons from its outer shell to the outer shells of each Fluorine atom. |
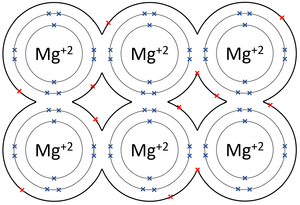
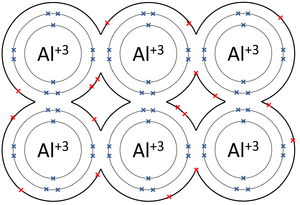
| The outer shells of the Magnesium atoms overlap allowing the two electrons in each outer shell to move freely between atoms. | The outer shells of the Aluminium atoms overlap allowing the three electrons in each outer shell to move freely between atoms. |
Extra Information
References
AQA
- Bonding, page 163, GCSE Combined Science Trilogy 1, Hodder, AQA
- Bonding, pages 47-8, GCSE Chemistry, Hodder, AQA
- Bonding; covalent, pages 154, 156-7, GCSE Combined Science Trilogy 1, Hodder, AQA
- Bonding; covalent, pages 41-2, GCSE Chemistry, Hodder, AQA
- Bonding; ionic, page 149, GCSE Combined Science Trilogy 1, Hodder, AQA
- Bonding; ionic, page 34, GCSE Chemistry, Hodder, AQA
- Bonding; metallic, page 45, GCSE Chemistry, Hodder, AQA
- Bonding; metallic, pages 160-1, GCSE Combined Science Trilogy 1, Hodder, AQA