Difference between revisions of "Nitrogen"
| Line 40: | Line 40: | ||
: [[Nitrogen]] is [[gas]] at [[STP|standard temperature and pressure]]. | : [[Nitrogen]] is [[gas]] at [[STP|standard temperature and pressure]]. | ||
: [[Nitrogen]] rarely [[Chemical Reaction|reacts]] with other [[chemical]]s. | : [[Nitrogen]] rarely [[Chemical Reaction|reacts]] with other [[chemical]]s. | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Nitrogen, pages 34, 65, 211, 215, 217, 219, 294-5, 344-5, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158770/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158770&linkCode=as2&tag=nrjc-21&linkId=ec31595e720e1529e49876c3866fff6e ''Nitrogen, pages 59, 287, GCSE Physics; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Nitrogen; dinitrogen tetroxide, page 214, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Nitrogen; dioxide, page 214, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Nitrogen; oxides (NOX) as pollutants, pages 312-5, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
Revision as of 13:39, 10 November 2019
Contents
Key Stage 2
Meaning
Key Stage 3
Meaning
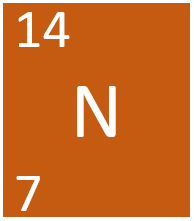
Nitrogen is a Group 5 non-metal element, on the Periodic Table, with an atomic number of 7.
About Nitrogen
- Nitrogen makes up 78% of the air.
- Nitrogen has the chemical symbol N.
Molecular Structure
- The chemical formula for a Nitrogen molecule is N2.
Atomic Structure
- Nitrogen has 7 protons and 7 neutrons in its nucleus giving it an atomic number of 7 and a atomic mass of 14.
- Nitrogen is in Period 2 of the Periodic Table because it has 2 electron shells.
Properties
Key Stage 4
Meaning
Nitrogen is a Group 5 non-metal element, on the Periodic Table, with 7 protons in the nucleus.
About Nitrogen
- Nitrogen makes up 78% of the air.
- Nitrogen has the chemical symbol N.
Molecular Structure
- The chemical formula for a Nitrogen molecule is N2.
- Nitrogen forms a covalent bond with another Nitrogen atom to produce a simple covalent molecule.
Atomic Structure
- The most common stable isotope of Nitrogen has 7 neutrons in its nucleus giving it an atomic mass of 14.
- Nitrogen is in Period 2 of the Periodic Table because it has 2 electron shells.
- Nitrogen has 5 electrons in its outer shell and needs 3 more electrons to get a full outer shell so it can form 3 bonds with other atoms.
Properties
References
AQA
- Nitrogen, pages 34, 65, 211, 215, 217, 219, 294-5, 344-5, GCSE Chemistry; Student Book, Collins, AQA
- Nitrogen, pages 59, 287, GCSE Physics; Student Book, Collins, AQA
- Nitrogen; dinitrogen tetroxide, page 214, GCSE Chemistry; Student Book, Collins, AQA
- Nitrogen; dioxide, page 214, GCSE Chemistry; Student Book, Collins, AQA
- Nitrogen; oxides (NOX) as pollutants, pages 312-5, GCSE Chemistry; Student Book, Collins, AQA