Difference between revisions of "Nitrogen"
| Line 4: | Line 4: | ||
==Key Stage 3== | ==Key Stage 3== | ||
| + | [[File:NitrogenSymbol1.png|right|300px|thumb|The [[Chemical Symbol|chemical symbol]] for [[Nitrogen]].]] | ||
| + | [[File:Nitrgon2dWK.PNG|right|200px|thumb|A 2 dimensional representation of a [[Nitrogen]] [[atom]] with 7 [[proton]]s and 7 [[neutron]]s in the [[Atomic Nucleus|nucleus]] and 7 [[electron]]s orbiting the [[Atomic Nucleus|nucleus]].]] | ||
===Meaning=== | ===Meaning=== | ||
| − | + | ||
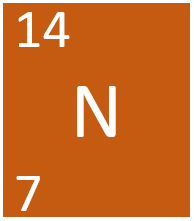
[[Nitrogen]] is a [[Group 5]] [[non-metal]] [[element]], on the [[Periodic Table]], with an [[Atomic Number|atomic number]] of 7. | [[Nitrogen]] is a [[Group 5]] [[non-metal]] [[element]], on the [[Periodic Table]], with an [[Atomic Number|atomic number]] of 7. | ||
Revision as of 14:01, 12 March 2020
Contents
Key Stage 2
Meaning
Key Stage 3
Meaning
Nitrogen is a Group 5 non-metal element, on the Periodic Table, with an atomic number of 7.
About Nitrogen
- Nitrogen makes up 78% of the air.
- Nitrogen has the chemical symbol N.
Molecular Structure
- The chemical formula for a Nitrogen molecule is N2.
Atomic Structure
- Nitrogen has 7 protons and 7 neutrons in its nucleus giving it an atomic number of 7 and a atomic mass of 14.
- Nitrogen is in Period 2 of the Periodic Table because it has 2 electron shells.
Properties
Key Stage 4
Meaning
Nitrogen is a Group 5 non-metal element, on the Periodic Table, with 7 protons in the nucleus.
About Nitrogen
- Nitrogen makes up 78% of the air.
- Nitrogen has the chemical symbol N.
Molecular Structure
- The chemical formula for a Nitrogen molecule is N2.
- Nitrogen forms a covalent bond with another Nitrogen atom to produce a simple covalent molecule.
Atomic Structure
- The most common stable isotope of Nitrogen has 7 neutrons in its nucleus giving it an atomic mass of 14.
- Nitrogen is in Period 2 of the Periodic Table because it has 2 electron shells.
- Nitrogen has 5 electrons in its outer shell and needs 3 more electrons to get a full outer shell so it can form 3 bonds with other atoms.
Properties
References
AQA
- Nitrogen, pages 34, 65, 211, 215, 217, 219, 294-5, 344-5, GCSE Chemistry; Student Book, Collins, AQA
- Nitrogen, pages 59, 287, GCSE Physics; Student Book, Collins, AQA
- Nitrogen; dinitrogen tetroxide, page 214, GCSE Chemistry; Student Book, Collins, AQA
- Nitrogen; dioxide, page 214, GCSE Chemistry; Student Book, Collins, AQA
- Nitrogen; oxides (NOX) as pollutants, pages 312-5, GCSE Chemistry; Student Book, Collins, AQA