Difference between revisions of "Oxygen"
| Line 49: | Line 49: | ||
:[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Oxygen, pages 184, 194-195, 197, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Oxygen, pages 184, 194-195, 197, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
:[https://www.amazon.co.uk/gp/product/0198359373/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359373&linkCode=as2&tag=nrjc-21&linkId=952a73bbb09d222ecc4b50d200679849 ''Oxygen, pages 52, 61, 124, 282, 288-289, GCSE Biology; Third Edition, Oxford University Press, AQA ''] | :[https://www.amazon.co.uk/gp/product/0198359373/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359373&linkCode=as2&tag=nrjc-21&linkId=952a73bbb09d222ecc4b50d200679849 ''Oxygen, pages 52, 61, 124, 282, 288-289, GCSE Biology; Third Edition, Oxford University Press, AQA ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359810/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359810&linkCode=as2&tag=nrjc-21&linkId=d768d99f1a06f7c12fab40e5aef85a55 ''Oxygen, pages 42, 49, 191, 236, 259, Gateway GCSE Biology, Oxford, OCR ''] | ||
Revision as of 15:06, 17 December 2019
Contents
Key Stage 2
Meaning
Key Stage 3
Meaning
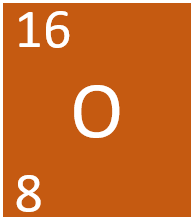
Oxygen is a Group 6 non-metal element, on the Periodic Table, with an atomic number of 8.
About Oxygen
- Oxygen makes up 21% of the air.
- Oxygen has the chemical symbol O.
Molecular Structure
- The chemical formula for a Oxygen molecule is O2.
Atomic Structure
- Oxygen has 8 protons and 8 neutrons in its nucleus giving it an atomic number of 8 and a atomic mass of 16.
- Oxygen is in Period 2 of the Periodic Table because it has 2 electron shells.
Properties
- Oxygen is a gas at room temperature.
Key Stage 4
Meaning
Oxygen is a Group 6 non-metal element, on the Periodic Table, with 8 protons in the nucleus.
About Oxygen
- Oxygen makes up 21% of the air.
- Oxygen has the chemical symbol O.
Molecular Structure
- The chemical formula for an Oxygen molecule is O2.
- Oxygen forms a covalent bond with another Oxygen atom to produce a simple covalent molecule.
Atomic Structure
- The most common stable isotope of Oxygen has 8 neutrons in its nucleus giving it an atomic mass of 16.
- Oxygen is in Period 2 of the Periodic Table because it has 2 electron shells.
- Oxygen has 6 electrons in its outer shell and needs 2 more to get a full outer shell so it can form 2 bonds with other atoms.
Properties
Testing for Oxygen
- Collect the gas in a test tube.
- Place a glowing splint over the mouth of the test tube.
- If the splint relights then the gas is Oxygen.
References
AQA
- Oxygen, page 287, GCSE Physics; Student Book, Collins, AQA
- Oxygen, pages 107, 184-5, 213, 292-5, GCSE Chemistry; Student Book, Collins, AQA
- Oxygen, pages 184, 194-195, 197, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Oxygen, pages 52, 61, 124, 282, 288-289, GCSE Biology; Third Edition, Oxford University Press, AQA