Contents
Key Stage 2
Meaning
Key Stage 3
Meaning
Silicon is a Group 4 metalloid element, on the Periodic Table, with an atomic number of 14.
About Silicon
- Silicon has the chemical symbol Si.
Atomic Structure
- Silicon as 14 protons and 14 neutrons in its nucleus giving it an Atomic Number of 14 and an atomic mass of 28.
- Silicon is in Period 3 of the Periodic Table because it has 3 electron shells.
Properties
- Silicon is a shiny solid at room temperature.
Key Stage 4
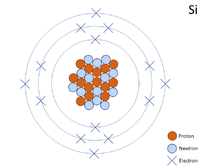
A 2 dimensional representation of the Bohr Model of a Silicon-28 isotope with 14 protons and 14 neutrons in the nucleus and 2 electrons in the first shell, 8 in the second and 4 in the outer shell.
Meaning
Silicon is a Group 4 metalloid element, on the Periodic Table, with 14 protons in the nucleus.
About Silicon
- Silicon has the chemical symbol Si.
Atomic Structure
- The most stable isotope of Silicon has 14 neutrons in its nucleus giving it an atomic mass of 28.
- Silicon is in Period 3 of the Periodic Table because it has 3 electron shells.
Properties
- Silicon is a metalloid element so in some conditions it is an electrical conductor.
- Silicon is a shiny solid at standard temperature and pressure and has a high melting point.
