Difference between revisions of "Electrical Conductor"
| (13 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
==Key Stage 2== | ==Key Stage 2== | ||
===Meaning=== | ===Meaning=== | ||
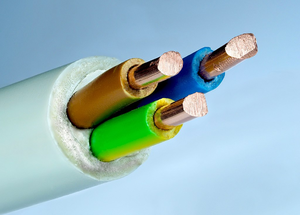
| − | An '''electrical conductor''' is a [[material]] that allows [[electricity]] to flow through | + | [[File:Wire3Core.png|right|300px|thumb|[[Wire|Wires]] have [[metal]] inside them because [[metal]] makes a good '''electrical conductor'''.]] |
| − | it easily. | + | An '''electrical conductor''' is a [[material]] that allows [[electricity]] to flow through it easily. |
: Singular [[Noun]]: '''Electrical conductor''' | : Singular [[Noun]]: '''Electrical conductor''' | ||
| Line 11: | Line 11: | ||
===About Electrical Conductors=== | ===About Electrical Conductors=== | ||
: [[Metal|Metals]] make good '''electrical conductors'''. | : [[Metal|Metals]] make good '''electrical conductors'''. | ||
| − | : [[ | + | : [[Plastic (Material)|Plastic]], [[rubber]] and the [[air]] make bad '''electrical conductors'''. |
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:MetalSword.png|center|200px]] | ||
| + | |[[File:Sea.png|center|200px]] | ||
| + | |- | ||
| + | |[[Metal]] is a good '''electrical conductor'''. | ||
| + | |[[Brine|Salty water]] is an '''electrical conductor'''. | ||
| + | |} | ||
| + | |||
| + | ==Key Stage 3== | ||
| + | ===Meaning=== | ||
| + | An '''electrical conductor''' is a [[material]] with a very low [[Electrical Resistance|resistance]] to the flow of [[electricity]]. | ||
| + | |||
| + | ===About Electrical Conductors=== | ||
| + | : [[Metal]] [[element]]s are good '''electrical conductors'''. | ||
| + | : [[Metal]]s make good '''conductors''' because they have [[Delocalised Electrons|free electrons]] that can move around the [[metal]]. | ||
| + | : [[Non-metal]] [[element]]s are usually poor '''electrical conductors'''. [[Carbon]] in the form of [[graphite]] is an exception to this. | ||
| + | : [[Salt]]s that are [[molten]] or [[dissolve]]d in [[water]] are '''electrical conductors'''. | ||
| + | : [[Salt]]s make good '''conductors''' when the [[ion]]s are free to move through the [[substance]]. | ||
| + | : To determine if an [[object]] is a good '''electrical conductor''' the [[object]] can be added to a [[circuit]]. If a [[Electrical Current|current]] flows then it is a good '''conductor'''. | ||
| + | : To compare the '''[[Electrical Conductivity|conductivity]]''' of different [[object]]s an [[ammeter]] can be added to the [[circuit]]. The higher the [[Electrical Current|current]] the better the [[object]] is at '''conducting'''. | ||
| + | |||
| + | ==Key Stage 4== | ||
| + | ===Meaning=== | ||
| + | An '''electrical conductor''' is a [[material]] with a very low [[Electrical Resistance|resistance]] to the flow of [[electricity]]. | ||
| + | |||
| + | ===About Electrical Conductors=== | ||
| + | : [[Metal]] [[element]]s are good '''electrical conductors'''. | ||
| + | : [[Metal]]s make good '''conductors''' due to the [[Metallic Bond|metallic bonds]] in which a sea [[Delocalised Electrons|delocalised electrons]] is free to move past a [[lattice]] of [[Positive Ion|positive]] [[metal]] [[ion]]s. | ||
| + | : [[Non-metal]] [[element]]s are usually poor '''electrical conductors''' as [[electron]]s are not free to move from one [[atom]] to another. [[Carbon]] in the form of [[graphite]] is an exception to this. | ||
| + | : [[Salt]]s that are [[molten]] or [[dissolve]]d in [[water]] are '''electrical conductors'''. | ||
| + | : [[Salt]]s make good '''conductors''' when the [[ion]]s are free to move through the [[substance]]. | ||
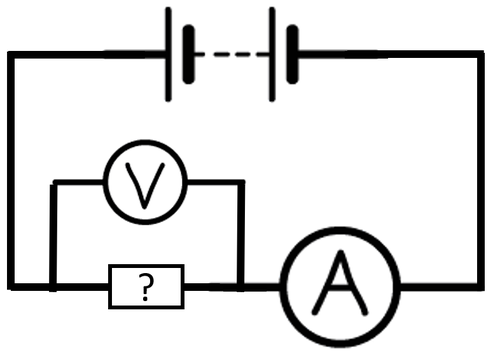
| + | : To determine if an [[object]] is a good '''electrical conductor''' the [[object]] can be added to a [[circuit]]. If the [[ratio]] of [[Potential Difference|potential difference]] to [[Electrical Current|current]] is low then it is a good '''conductor'''. | ||
| + | : To compare the '''[[Electrical Conductivity|conductivity]]''' of [[Electrical Component|components]] in a [[circuit]] an [[ammeter]] can be added in [[Series Circuit|series]] with the [[Electrical Component|component]] and a [[voltmeter]] in [[Parallel Circuit|parallel]] to the [[Electrical Component|component]]. The smaller the [[Electrical Resistance|resistance]] the better the [[object]] is at '''conducting'''. | ||
| + | |||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:ResistanceComponent.png|center|500px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |A [[Circuit Diagram|circuit diagram]] showing how to find the [[Electrical Resistance|resistance]] of an unknown [[Electrical Component|component]]. | ||
| + | |} | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/0008158770/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158770&linkCode=as2&tag=nrjc-21&linkId=ec31595e720e1529e49876c3866fff6e ''Conductor (electrical), page 48, GCSE Physics; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945970/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945970&linkCode=as2&tag=nrjc-21&linkId=a120d24dcc7cc7a58192069a3aafc1d2 ''Conductors; electrical, page 99, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA ''] | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782945970/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945970&linkCode=as2&tag=nrjc-21&linkId=a120d24dcc7cc7a58192069a3aafc1d2 ''Electrical conductors, page 99, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA ''] | ||
Latest revision as of 10:42, 4 November 2019
Contents
Key Stage 2
Meaning
An electrical conductor is a material that allows electricity to flow through it easily.
- Singular Noun: Electrical conductor
- Plural Noun: Electrical conductors
- Verb: To electrically conduct
- Adjective: Electrically conductive
About Electrical Conductors
| Metal is a good electrical conductor. | Salty water is an electrical conductor. |
Key Stage 3
Meaning
An electrical conductor is a material with a very low resistance to the flow of electricity.
About Electrical Conductors
- Metal elements are good electrical conductors.
- Metals make good conductors because they have free electrons that can move around the metal.
- Non-metal elements are usually poor electrical conductors. Carbon in the form of graphite is an exception to this.
- Salts that are molten or dissolved in water are electrical conductors.
- Salts make good conductors when the ions are free to move through the substance.
- To determine if an object is a good electrical conductor the object can be added to a circuit. If a current flows then it is a good conductor.
- To compare the conductivity of different objects an ammeter can be added to the circuit. The higher the current the better the object is at conducting.
Key Stage 4
Meaning
An electrical conductor is a material with a very low resistance to the flow of electricity.
About Electrical Conductors
- Metal elements are good electrical conductors.
- Metals make good conductors due to the metallic bonds in which a sea delocalised electrons is free to move past a lattice of positive metal ions.
- Non-metal elements are usually poor electrical conductors as electrons are not free to move from one atom to another. Carbon in the form of graphite is an exception to this.
- Salts that are molten or dissolved in water are electrical conductors.
- Salts make good conductors when the ions are free to move through the substance.
- To determine if an object is a good electrical conductor the object can be added to a circuit. If the ratio of potential difference to current is low then it is a good conductor.
- To compare the conductivity of components in a circuit an ammeter can be added in series with the component and a voltmeter in parallel to the component. The smaller the resistance the better the object is at conducting.
| A circuit diagram showing how to find the resistance of an unknown component. |
References
AQA
- Conductor (electrical), page 48, GCSE Physics; Student Book, Collins, AQA
- Conductors; electrical, page 99, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA