Difference between revisions of "Ionic Bond"
(Created page with "==Key Stage 4== ===Meaning=== An '''ionic bond''' is a type of chemical bond in which atoms form ions be exchanging electrons. ===About Ionic Bo...") |
(→Examples) |
||
| Line 12: | Line 12: | ||
{| class="wikitable" | {| class="wikitable" | ||
|[[File:LithiumFluorideDotandCrossDiagram.png|center|200px]] | |[[File:LithiumFluorideDotandCrossDiagram.png|center|200px]] | ||
| − | |[[File: | + | |[[File:MagnesiumOxideDotandCrossDiagram.png|center|200px]] |
| − | |[[File: | + | |[[File:MagnesiumChlorideDotandCrossDiagram.png|center|200px]] |
|- | |- | ||
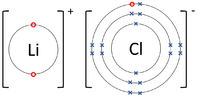
| style="height:20px; width:200px; text-align:center;" |The [[Lithium]] [[atom]] loses an [[electron]] to get a full [[Outer Shell|outer shell]] becoming a Li<sup>+1</sup> [[ion]] while the [[Fluorine]] [[atom]] gains an [[electron]] to get a full [[Outer Shell|outer shell]] becoming a F<sup>-1</sup> [[ion]]. | | style="height:20px; width:200px; text-align:center;" |The [[Lithium]] [[atom]] loses an [[electron]] to get a full [[Outer Shell|outer shell]] becoming a Li<sup>+1</sup> [[ion]] while the [[Fluorine]] [[atom]] gains an [[electron]] to get a full [[Outer Shell|outer shell]] becoming a F<sup>-1</sup> [[ion]]. | ||
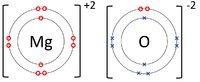
| − | | style="height:20px; width:200px; text-align:center;" |The [[ | + | | style="height:20px; width:200px; text-align:center;" |The [[Magnsium]] [[atom]] loses two [[electron]]s to get a full [[Outer Shell|outer shell]] becoming a Mg<sup>+2</sup> [[ion]] while the [[Oxygen]] [[atom]] gains two [[electron]]s to get a full [[Outer Shell|outer shell]] becoming an O<sup>-2</sup> [[ion]]. |
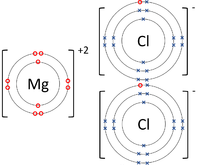
| style="height:20px; width:200px; text-align:center;" |The [[Magnesium]] [[atom]] loses two [[electron]]s to get a full [[Outer Shell|outer shell]] becoming a Mg<sup>+2</sup> [[ion]] while the two [[Chlorine]] [[atom]]s each gain an [[electron]] to get a full [[Outer Shell|outer shell]] becoming a Cl<sup>-1</sup> [[ion]]s. | | style="height:20px; width:200px; text-align:center;" |The [[Magnesium]] [[atom]] loses two [[electron]]s to get a full [[Outer Shell|outer shell]] becoming a Mg<sup>+2</sup> [[ion]] while the two [[Chlorine]] [[atom]]s each gain an [[electron]] to get a full [[Outer Shell|outer shell]] becoming a Cl<sup>-1</sup> [[ion]]s. | ||
|} | |} | ||
Revision as of 11:38, 28 December 2018
Key Stage 4
Meaning
An ionic bond is a type of chemical bond in which atoms form ions be exchanging electrons.
About Ionic Bonds
- Atoms are more chemically stable when their outer shell is full of electrons. One way atoms can have a full outer shell is either giving away an electron or gaining an electron from another atom.
- When an electron has been transferred from one atom to another the atoms become charged and form ions. These ions experience an electrostatic force of attraction. This is known as an ionic bond.
- Ionic bonds usually happen between a metal and a non-metal element when a metal atom loses one or more electrons and the non-metal atom gains one or more electrons.
- Ionic bonds can be represented by a Dot and Cross Diagram to show how the electrons are transferred from one atom to another.
Examples
| The Lithium atom loses an electron to get a full outer shell becoming a Li+1 ion while the Fluorine atom gains an electron to get a full outer shell becoming a F-1 ion. | The Magnsium atom loses two electrons to get a full outer shell becoming a Mg+2 ion while the Oxygen atom gains two electrons to get a full outer shell becoming an O-2 ion. | The Magnesium atom loses two electrons to get a full outer shell becoming a Mg+2 ion while the two Chlorine atoms each gain an electron to get a full outer shell becoming a Cl-1 ions. |