Difference between revisions of "Beryllium"
| Line 9: | Line 9: | ||
: [[Beryllium]] has the [[Chemical Formula|chemical formula]] [[Beryllium|Be]]. | : [[Beryllium]] has the [[Chemical Formula|chemical formula]] [[Beryllium|Be]]. | ||
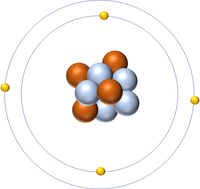
: [[Beryllium]] as 4 [[proton]]s and 5 [[neutron]]s in its [[Atomic Nucleus|nucleus]] giving it an [[Atomic Number]] of 4 and an [[Relative Atomic Mass|atomic mass]] of 9. | : [[Beryllium]] as 4 [[proton]]s and 5 [[neutron]]s in its [[Atomic Nucleus|nucleus]] giving it an [[Atomic Number]] of 4 and an [[Relative Atomic Mass|atomic mass]] of 9. | ||
| − | : [[Beryllium]] is the least [[Reactivity|reactive]] | + | : [[Beryllium]] is the least [[Reactivity|reactive]] [[Alkali Earth Metal|alkali earth metal]]. |
: [[Beryllium]] is more [[Reactivity|reactive]] than [[Carbon]] on the [[Reactivity Series|reactivity series]] so it must be [[Extraction of Metals|extracted]] from its [[ore]] using [[electrolysis]]. | : [[Beryllium]] is more [[Reactivity|reactive]] than [[Carbon]] on the [[Reactivity Series|reactivity series]] so it must be [[Extraction of Metals|extracted]] from its [[ore]] using [[electrolysis]]. | ||
: [[Beryllium]] [[Chemical Reaction|reacts]] strongly with [[steam]] to produce [[Hydrogen]] [[gas]] and [[Beryllium Hydroxide]]. | : [[Beryllium]] [[Chemical Reaction|reacts]] strongly with [[steam]] to produce [[Hydrogen]] [[gas]] and [[Beryllium Hydroxide]]. | ||
| Line 21: | Line 21: | ||
: [[Beryllium]] has the [[Chemical Formula|chemical formula]] [[Beryllium|Be]]. | : [[Beryllium]] has the [[Chemical Formula|chemical formula]] [[Beryllium|Be]]. | ||
: The most [[Stable Isotope|stable isotope]] of [[Beryllium]] has 5 [[neutron]]s in its [[Atomic Nucleus|nucleus]] giving it an [[Relative Atomic Mass|atomic mass]] of 9. | : The most [[Stable Isotope|stable isotope]] of [[Beryllium]] has 5 [[neutron]]s in its [[Atomic Nucleus|nucleus]] giving it an [[Relative Atomic Mass|atomic mass]] of 9. | ||
| − | : [[Beryllium]] is the least [[Reactivity|reactive]] | + | : [[Beryllium]] is the least [[Reactivity|reactive]] [[Alkali Earth Metal|alkali earth metal]]. |
: [[Beryllium]] is more [[Reactivity|reactive]] than [[Carbon]] on the [[Reactivity Series|reactivity series]] so it must be [[Extraction of Metals|extracted]] from its [[ore]] using [[electrolysis]]. | : [[Beryllium]] is more [[Reactivity|reactive]] than [[Carbon]] on the [[Reactivity Series|reactivity series]] so it must be [[Extraction of Metals|extracted]] from its [[ore]] using [[electrolysis]]. | ||
: [[Beryllium]] [[Chemical Reaction|reacts]] strongly with [[steam]] to produce [[Hydrogen]] [[gas]] and [[Beryllium Hydroxide]]. | : [[Beryllium]] [[Chemical Reaction|reacts]] strongly with [[steam]] to produce [[Hydrogen]] [[gas]] and [[Beryllium Hydroxide]]. | ||
Revision as of 14:37, 31 March 2019
Contents
Key Stage 2
Meaning
Key Stage 3
Meaning
Beryllium is a Group 2 element, on the Periodic Table, with an atomic number of 4.
About Beryllium
- Beryllium has the chemical formula Be.
- Beryllium as 4 protons and 5 neutrons in its nucleus giving it an Atomic Number of 4 and an atomic mass of 9.
- Beryllium is the least reactive alkali earth metal.
- Beryllium is more reactive than Carbon on the reactivity series so it must be extracted from its ore using electrolysis.
- Beryllium reacts strongly with steam to produce Hydrogen gas and Beryllium Hydroxide.
- Lithium is a solid at room temperature.
- An atom of Beryllium has only 2 electrons in its outer shell.
- Beryllium ions have lost 2 electrons to become positively charged.
Key Stage 4
Meaning
Beryllium is a Group 2 element, on the Periodic Table, with 4 protons in the nucleus.
About Beryllium
- Beryllium has the chemical formula Be.
- The most stable isotope of Beryllium has 5 neutrons in its nucleus giving it an atomic mass of 9.
- Beryllium is the least reactive alkali earth metal.
- Beryllium is more reactive than Carbon on the reactivity series so it must be extracted from its ore using electrolysis.
- Beryllium reacts strongly with steam to produce Hydrogen gas and Beryllium Hydroxide.
- Lithium is a solid at standard temperature and pressure.
- An atom of Beryllium has only 2 electrons in its outer shell.
- Beryllium ions have lost two electrons to become positively charged.
| Beryllium always has 4 protons. The most stable isotope has 5 neutrons. | |