Key Stage 3
Meaning
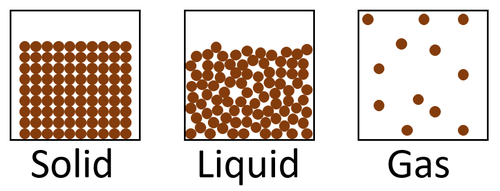
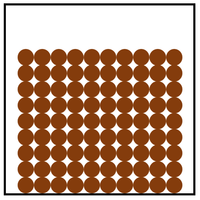
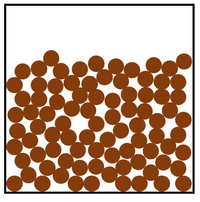
The particle model is a scientific theory that explains the properties of solids, liquids and gases by suggesting that all matter is made of particles, and that those particles behave differently in solids, liquids and gases.
| A diagram showing the particle model for solids, liquids and gases. |
About The Particle Model
- The particle model explains the properties of solids, liquids and gases.
- The particle model can explain changes of state.
- Evidence of the particle model can be shown by pouring 50ml of pure water and 50ml of pure ethanol into a measuring cylinder. The solution is only 97ml because ethanol molecules are bigger than water molecules so the water molecules fit between the ethanol molecules like pouring 50ml of sand and 50ml of marbles into the same container. It will not make 100ml.
- Evidence of the particle model can be shown by observing Brownian Motion.
Key Stage 4
Meaning
The particle model is a scientific theory that explains the properties of solids, liquids and gases by suggesting that all matter is made of particles, and that those particles behave differently in solids, liquids and gases.
About The Particle Model
- The particle model describes how the particles that make a solid, liquid or gas are arranged and how they move.
- In the particle model the particles are constantly moving and colliding with one another.
- The particles have kinetic energy which is passed on to each other during collisions.
| Diagram | Arrangement | Motion |
| In a solid the particles are in a regular arrangement and very close together. This is the most dense state of matter. | In a solid the particles vibrate around fixed positions. | |
| In a liquid the particles are in a random arrangement with small gaps between them. | In a liquid the particles can slide past one another. | |
| In a gas the particles are in a random arrangement and spread far apart from one another. This is the least dense state of matter. | In a gas the particles are free to move in all directions. |
Limitations of the Particle Model
- The particle model is not a complete explanation for the properties of a material. However, it is a useful approximation which can make predictions about the properties of solids, liquids and gases, that is not perfect.
- The particle model only explains the properties of solids, liquids and gases but not why different materials are solid, liquid or gas at different temperatures.
The problems with the particle model are that it makes several assumptions which are not always the case:
| Assumption | Reality | Problem |
| Particles are spheres. | Particles are often molecules whose shape is not a sphere, some of which are long chains of atoms. |
|
| There are no forces between particles. | There are forces between atoms and intermolecular forces between molecules. |
|
| The size of all particles in a substance is the same. | A substance can be made of more than one different particle which have different sizes. |
Chemical Reactions in the Particle Model
- During a chemical reaction atoms are rearranged into new compounds. The particle model explains how temperature and pressure affect the rate of chemical reactions.
- In the particle model the particles collide with one another.
- If particles collide with enough kinetic energy it can break the chemical bonds holding the atoms to each other in a molecule.
- When collisions have caused molecules to break apart they can join back together in a new arrangement.
Temperature and Chemical Reactions
- When the temperature of a substance is increased it causes the particles to move around faster, with greater kinetic energy.
- As temperature increases the particles collide more often and with greater kinetic energy.
- When particles collide more often it means a greater chance of the chemical bonds breaking and atoms rearranging into new compounds.
- When particles have a large kinetic energy during a collision they are more likely to have enough energy to break the chemical bonds holding atoms in the molecules together.
Pressure and Chemical Reactions
- When the pressure of a substance is increased it causes the particles collide more often.
- When particles collide more often it means a greater chance of the chemical bonds breaking and atoms rearranging into new compounds.
References
AQA
- Particle model, pages 106, 107, 113-115, GCSE Physics; The Complete 9-1 Course for AQA, CGP, AQA
- Particle model, pages 36-37, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Particle model, pages 68-9, GCSE Chemistry; Student Book, Collins, AQA
- Particle model, pages 96, 97, 103, 104, GCSE Combined Science Trilogy; Physics, CGP, AQA
- Particle; model, pages 82-5, 89-90, 96, 98, 175, GCSE Physics; Student Book, Collins, AQA
- Particle theory, pages 36, 37, GCSE Chemistry; The Revision Guide, CGP, AQA
- Particle theory, pages 97, 98, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Particle theory, pages 99, 100, GCSE Chemistry, CGP, AQA
Edexcel
- Particle model, pages 34, 35, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Particle model, pages 95-98, GCSE Chemistry, CGP, Edexcel
- Particle model, pages 97, 98, GCSE Combined Science; The Revision Guide, CGP, Edexcel
OCR
- Particle model, pages 18-21, Gateway GCSE Physics, Oxford, OCR
- Particle model, pages 18-23, 176-181, Gateway GCSE Chemistry, Oxford, OCR
- Particle model, pages 82, 107, 152, 155, Gateway GCSE Combined Science; The Revision Guide, CGP, OCR
- Particle theory, pages 12, 34, Gateway GCSE Chemistry; The Revision Guide, CGP, OCR
- Particle theory, pages 14, 17, 18, Gateway GCSE Physics; The Revision Guide, CGP, OCR
Beyond the Curriculum
This section delves deeper into the fascinating world of the particle model, offering insights beyond what's covered in the standard curriculum for Key Stage 3 and 4 in England and Wales. While the curriculum provides essential knowledge, there are intriguing facets of the particle model that are equally captivating.
Particle Waves
One of the remarkable aspects of particles is their wave-particle duality. This concept suggests that particles, such as electrons, can exhibit both particle-like and wave-like behaviours. It's like seeing a soccer ball behave as a wave instead of a solid object. This strange phenomenon is a fundamental principle of quantum mechanics and plays a crucial role in understanding the behaviour of particles at the subatomic level.
Quantum Tunneling
Imagine a tiny particle approaching a barrier that, according to classical physics, it shouldn't be able to pass through. However, in the quantum world, particles can "tunnel" through barriers. This phenomenon, known as quantum tunneling, is a testament to the peculiar behaviour of particles at the quantum scale. It has practical applications in electronics, where it enables the operation of devices like tunnel diodes.
Bose-Einstein Condensate
At extremely low temperatures, certain particles can condense into a unique state of matter known as a Bose-Einstein condensate. In this state, these particles lose their individuality and behave as a single quantum entity. It's as if a crowd of people suddenly starts moving in perfect unison, exhibiting the weird and wonderful nature of particles under extreme conditions.
Particle Physics Mysteries
The field of particle physics explores the fundamental building blocks of the universe. Some of the questions it seeks to answer go far beyond the curriculum, such as the search for dark matter and understanding the nature of cosmic rays. These mysteries push the boundaries of our knowledge about the particle world and the universe itself.
These captivating aspects of particle physics, while not covered in the standard curriculum, offer a glimpse into the exciting and mysterious realm of particles. They demonstrate that there's always more to explore and discover, even beyond what textbooks can teach.