Contents
Key Stage 2
Meaning
Key Stage 3
Meaning
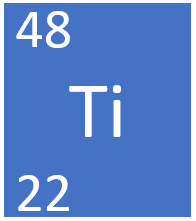
Titanium is a transition metal element, on the Periodic Table, with an atomic number of 22.
About Titanium
Molecular Structure
- Titanium has the chemical symbol Ti.
- Titanium atoms join together in large numbers to form a giant metal molecule.
Atomic Structure
- Titanium as 22 protons and 26 neutrons in its nucleus giving it an Atomic Number of 22 and an atomic mass of 48.
- Titanium is in Period 4 of the Periodic Table because it has 4 electron shells.
Properties
- Titanium is a metal element so it is a good thermal conductor and a good electrical conductor.
- Titanium is a shiny solid at room temperature.
- Titanium is malleable.
- Titanium is sonorous.
- Titanium is ductile.
Key Stage 4
Meaning
Titanium is a transition metal element, on the Periodic Table, with 22 protons in the nucleus.
About Titanium
Molecular Structure
- Titanium has the chemical formula Ti.
- Titanium atoms join together in a giant metallic structure.
Atomic Structure
- The most stable isotope of Titanium has 26 neutrons in its nucleus giving it an atomic mass of 48.
- Titanium is in Period 4 of the Periodic Table because it has 4 electron shells.
- Titanium loses electrons to form positive metal ions.
Properties
- Titanium forms ionic bonds with non-metals.
- Titanium is a metal element so it is a good thermal conductor and a good electrical conductor.
- Titanium is a shiny solid at standard temperature and pressure and has a high melting point.
- Titanium is malleable.
- Titanium is sonorous.
- Titanium is ductile.