Difference between revisions of "Group 7"
| Line 56: | Line 56: | ||
:[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Group 7, pages 61-63, GCSE Chemistry, CGP, AQA ''] | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Group 7, pages 61-63, GCSE Chemistry, CGP, AQA ''] | ||
:[https://www.amazon.co.uk/gp/product/178294639X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294639X&linkCode=as2&tag=nrjc-21&linkId=51599bb45a2bfaf7c1b6a978b2ca2616 ''Group 7, pages 61-63, GCSE Combined Science Trilogy; Chemistry, CGP, AQA ''] | :[https://www.amazon.co.uk/gp/product/178294639X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294639X&linkCode=as2&tag=nrjc-21&linkId=51599bb45a2bfaf7c1b6a978b2ca2616 ''Group 7, pages 61-63, GCSE Combined Science Trilogy; Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945598/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945598&linkCode=as2&tag=nrjc-21&linkId=ad276ad49df77ab4b40ab4fd0fe09979 ''Halogens, page 110, GCSE Combined Science; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851354/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851354&linkCode=as2&tag=nrjc-21&linkId=9012a0d354024419214fb3ad5ac44ba0 ''Halogens, pages 131-4, GCSE Combined Science Trilogy 1, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Halogens, pages 16-18, GCSE Chemistry, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945571/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945571&linkCode=as2&tag=nrjc-21&linkId=9e29fad914244909903e5e93f8a01d152 ''Halogens, pages 25, 79, GCSE Chemistry; The Revision Guide, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Halogens, pages 28-29, 31, 158, 188-189, GCSE Chemistry; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Halogens, pages 44-5, 46, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Halogens, pages 61-63, GCSE Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294639X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294639X&linkCode=as2&tag=nrjc-21&linkId=51599bb45a2bfaf7c1b6a978b2ca2616 ''Halogens, pages 61-63, GCSE Combined Science Trilogy; Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Halogens; reaction with alkenes, pages 178-9, GCSE Chemistry, Hodder, AQA ''] | ||
Revision as of 14:36, 6 November 2019
Contents
Key Stage 4
Meaning
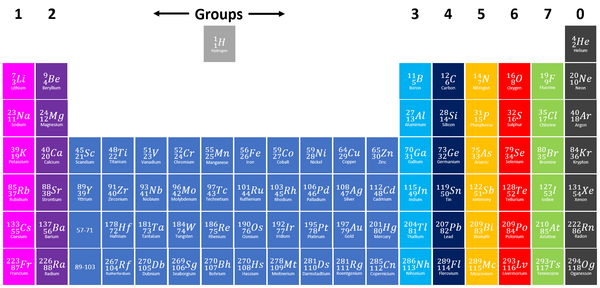
Group 7 elements, also known as Halogens on the Periodic Table are the elements which have 7 electrons in their outer shell.
| Group 7 elements are shown in green at the right of the Periodic Table. |
About the Halogens
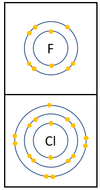
- The Halogens have similar chemical properties because they all have 7 electrons on their outer shell.
- Halogens all produce ions with a -1 relative charge because they gain an electron in chemical reactions.
The Halogens in order from most reactive to least reactive are:
Chemical Properties
- The reactivity of Halogens decreases as you go down the Periodic Table.
- Halogens all react strongly as bleaching agents.
- Halogens all produce acids when combined with Hydrogen.
- Halogens are toxic to bacteria and are used in disinfectants.
| In a chemical reaction an extra electron is added to the outer shell.
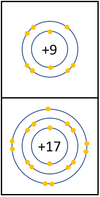
The reactivity decreases as you go down the group because:
|
Physical Properties
The physical properties of Halogens changes significantly as you go down the Periodic Table:
- Fluorine - A yellow gas at room temperature.
- Chlorine - A green gas at room temperature.
- Bromine - A brown liquid at room temperature.
- Iodine - A purple solid at room temperature.
- Astatine -A dark purple solid at room temperature.
- The density, melting point and boiling point all increase as you go down the Periodic Table.
References
AQA
- Group 7 (halogens), pages 131-4, GCSE Combined Science Trilogy 1, Hodder, AQA
- Group 7 (halogens), pages 44-5, 46, GCSE Chemistry; Student Book, Collins, AQA
- Group 7, page 110, GCSE Combined Science; The Revision Guide, CGP, AQA
- Group 7, page 25, GCSE Chemistry; The Revision Guide, CGP, AQA
- Group 7, pages 16-18, GCSE Chemistry, Hodder, AQA
- Group 7, pages 28-29, 31, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Group 7, pages 61-63, GCSE Chemistry, CGP, AQA
- Group 7, pages 61-63, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Halogens, page 110, GCSE Combined Science; The Revision Guide, CGP, AQA
- Halogens, pages 131-4, GCSE Combined Science Trilogy 1, Hodder, AQA
- Halogens, pages 16-18, GCSE Chemistry, Hodder, AQA
- Halogens, pages 25, 79, GCSE Chemistry; The Revision Guide, CGP, AQA
- Halogens, pages 28-29, 31, 158, 188-189, GCSE Chemistry; Third Edition, Oxford University Press, AQA
- Halogens, pages 44-5, 46, GCSE Chemistry; Student Book, Collins, AQA
- Halogens, pages 61-63, GCSE Chemistry, CGP, AQA
- Halogens, pages 61-63, GCSE Combined Science Trilogy; Chemistry, CGP, AQA
- Halogens; reaction with alkenes, pages 178-9, GCSE Chemistry, Hodder, AQA