Difference between revisions of "Aluminium"
(Created page with "Aluminium is a solid (at room temperature) metal element with 13 protons in its nucleus.") |
|||
| (9 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[Aluminium]] is a [[solid]] | + | ==Key Stage 2== |
| + | ===Meaning=== | ||
| + | [[Aluminium]] is a [[metal]]. | ||
| + | ==Key Stage 3== | ||
| + | [[File:AluminiumSymbol1.png|right|300px|thumb|The [[Chemical Symbol|chemical symbol]] for [[Aluminium]].]] | ||
| + | [[File:Al-27_WK.PNG|right|200px|thumb|A 2 dimensional representation of a [[Aluminium]] [[atom]] with 13 [[proton]]s and 14 [[neutron]]s in the [[Atomic Nucleus|nucleus]] and 13 [[electron]]s orbiting the [[Atomic Nucleus|nucleus]].]] | ||
| + | ===Meaning=== | ||
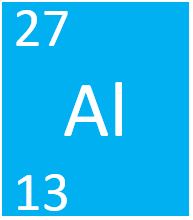
| + | [[Aluminium]] is a [[Group 3]] [[metal]] [[element]], on the [[Periodic Table]], with an [[Atomic Number|atomic number]] of 13. | ||
| + | |||
| + | ===About Aluminium=== | ||
| + | ====Molecular Structure==== | ||
| + | : [[Aluminium]] has the [[Chemical Symbol|chemical symbol]] [[Aluminium|Al]]. | ||
| + | : [[Aluminium]] [[atom]]s join together in large numbers to form a giant [[metal]] [[molecule]]. | ||
| + | ====Atomic Structure==== | ||
| + | : [[Aluminium]] as 13 [[proton]]s and 14 [[neutron]]s in its [[Atomic Nucleus|nucleus]] giving it an [[Atomic Number]] of 13 and an [[Relative Atomic Mass|atomic mass]] of 27. | ||
| + | : [[Aluminium]] is in [[Period]] 3 of the [[Periodic Table]] because it has 3 [[Electron Orbital|electron shells]]. | ||
| + | ====Properties==== | ||
| + | : [[Aluminium]] is a [[metal]] [[element]] so it is a good [[Thermal Conductor|thermal conductor]] and a good [[Electrical Conductor|electrical conductor]]. | ||
| + | : [[Aluminium]] is a shiny [[solid]] at [[STP|room temperature]]. | ||
| + | : [[Aluminium]] is [[malleable]]. | ||
| + | : [[Aluminium]] is [[sonorous]]. | ||
| + | : [[Aluminium]] is [[ductile]]. | ||
| + | |||
| + | ==Key Stage 4== | ||
| + | [[File:AlKS4.PNG|right|200px|thumb|The [[Chemical Symbol|chemical symbol]] for [[Aluminium]].]] | ||
| + | [[File:Al-27_WK.PNG|right|200px|thumb|A 2 dimensional representation of the [[Bohr Model]] of a [[Aluminium]]-27 [[isotope]] with 13 [[proton]]s and 14 [[neutron]]s in the [[Atomic Nucleus|nucleus]] and 2 [[electron]]s in the first [[Electron Orbital|shell]], 8 in the second and 3 in the [[Outer Shell|outer shell]].]] | ||
| + | ===Meaning=== | ||
| + | [[Aluminium]] is a [[Group 3]] [[metal]] [[element]], on the [[Periodic Table]], with 13 [[proton]]s in the [[Atomic Nucleus|nucleus]]. | ||
| + | ===About Aluminium=== | ||
| + | ====Molecular Structure==== | ||
| + | : [[Aluminium]] has the [[Chemical Formula|chemical formula]] [[Aluminium|Al]]. | ||
| + | : [[Aluminium]] [[atom]]s join together in a [[Giant Metallic Structure|giant metallic structure]]. | ||
| + | ====Atomic Structure==== | ||
| + | : The most [[Stable Isotope|stable isotope]] of [[Aluminium]] has 14 [[neutron]]s in its [[Atomic Nucleus|nucleus]] giving it an [[Relative Atomic Mass|atomic mass]] of 27. | ||
| + | : [[Aluminium]] is in [[Period]] 3 of the [[Periodic Table]] because it has 3 [[Electron Orbital|electron shells]]. | ||
| + | : [[Aluminium]] loses [[electron]]s to form [[Positive Charge|positive]] [[Metal Ion|metal ions]]. | ||
| + | ====Properties==== | ||
| + | : [[Aluminium]] forms [[Ionic Bond|ionic bonds]] with [[non-metal]]s. | ||
| + | : [[Aluminium]] is a [[metal]] [[element]] so it is a good [[Thermal Conductor|thermal conductor]] and a good [[Electrical Conductor|electrical conductor]]. | ||
| + | : [[Aluminium]] is a shiny [[solid]] at [[STP|standard temperature and pressure]] and has a high [[Melting Point|melting point]]. | ||
| + | : [[Aluminium]] is [[malleable]]. | ||
| + | : [[Aluminium]] is [[sonorous]]. | ||
| + | : [[Aluminium]] is [[ductile]]. | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | :[https://www.amazon.co.uk/gp/product/019835939X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=019835939X&linkCode=as2&tag=nrjc-21&linkId=57e96876985fc39b1a3d8a3e3dc238b6 ''Aluminium foil, page 32, GCSE Physics; Third Edition, Oxford University Press, AQA'] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851354/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851354&linkCode=as2&tag=nrjc-21&linkId=9012a0d354024419214fb3ad5ac44ba0 ''Aluminium, extraction from ore, pages 220-1, GCSE Combined Science Trilogy 1, Hodder, AQA'] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Aluminium, extraction of, page 119, GCSE Chemistry, Hodder, AQA'] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294639X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294639X&linkCode=as2&tag=nrjc-21&linkId=51599bb45a2bfaf7c1b6a978b2ca2616 ''Aluminium, page 144, GCSE Combined Science Trilogy; Chemistry, CGP, AQA'] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359381/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359381&linkCode=as2&tag=nrjc-21&linkId=47c8d1ae58d8b3a5e2094cd447154558 ''Aluminium, pages 106-107, 216, 222, GCSE Chemistry; Third Edition, Oxford University Press, AQA'] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Aluminium, pages 169, 286, 287, GCSE Chemistry, CGP, AQA'] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Aluminium, pages 26, 36, 79, 133, 158, 339-41, GCSE Chemistry; Student Book, Collins, AQA'] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Aluminium; extraction by electrolysis, page 158-9, GCSE Chemistry; Student Book, Collins, AQA'] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Aluminium; ore, page 133, GCSE Chemistry; Student Book, Collins, AQA'] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Aluminium; oxide, pages 133, 158-9, 269, GCSE Chemistry; Student Book, Collins, AQA'] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Aluminium; recycling, page 158, GCSE Chemistry; Student Book, Collins, AQA'] | ||
| + | |||
| + | ====OCR==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Aluminium, pages 210-211, 214, 217, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
Latest revision as of 13:59, 19 February 2021
Contents
Key Stage 2
Meaning
Key Stage 3
Meaning
Aluminium is a Group 3 metal element, on the Periodic Table, with an atomic number of 13.
About Aluminium
Molecular Structure
- Aluminium has the chemical symbol Al.
- Aluminium atoms join together in large numbers to form a giant metal molecule.
Atomic Structure
- Aluminium as 13 protons and 14 neutrons in its nucleus giving it an Atomic Number of 13 and an atomic mass of 27.
- Aluminium is in Period 3 of the Periodic Table because it has 3 electron shells.
Properties
- Aluminium is a metal element so it is a good thermal conductor and a good electrical conductor.
- Aluminium is a shiny solid at room temperature.
- Aluminium is malleable.
- Aluminium is sonorous.
- Aluminium is ductile.
Key Stage 4
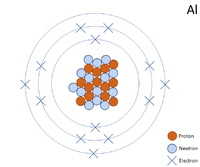
A 2 dimensional representation of the Bohr Model of a Aluminium-27 isotope with 13 protons and 14 neutrons in the nucleus and 2 electrons in the first shell, 8 in the second and 3 in the outer shell.
Meaning
Aluminium is a Group 3 metal element, on the Periodic Table, with 13 protons in the nucleus.
About Aluminium
Molecular Structure
- Aluminium has the chemical formula Al.
- Aluminium atoms join together in a giant metallic structure.
Atomic Structure
- The most stable isotope of Aluminium has 14 neutrons in its nucleus giving it an atomic mass of 27.
- Aluminium is in Period 3 of the Periodic Table because it has 3 electron shells.
- Aluminium loses electrons to form positive metal ions.
Properties
- Aluminium forms ionic bonds with non-metals.
- Aluminium is a metal element so it is a good thermal conductor and a good electrical conductor.
- Aluminium is a shiny solid at standard temperature and pressure and has a high melting point.
- Aluminium is malleable.
- Aluminium is sonorous.
- Aluminium is ductile.
References
AQA
- Aluminium foil, page 32, GCSE Physics; Third Edition, Oxford University Press, AQA'
- Aluminium, extraction from ore, pages 220-1, GCSE Combined Science Trilogy 1, Hodder, AQA'
- Aluminium, extraction of, page 119, GCSE Chemistry, Hodder, AQA'
- Aluminium, page 144, GCSE Combined Science Trilogy; Chemistry, CGP, AQA'
- Aluminium, pages 106-107, 216, 222, GCSE Chemistry; Third Edition, Oxford University Press, AQA'
- Aluminium, pages 169, 286, 287, GCSE Chemistry, CGP, AQA'
- Aluminium, pages 26, 36, 79, 133, 158, 339-41, GCSE Chemistry; Student Book, Collins, AQA'
- Aluminium; extraction by electrolysis, page 158-9, GCSE Chemistry; Student Book, Collins, AQA'
- Aluminium; ore, page 133, GCSE Chemistry; Student Book, Collins, AQA'
- Aluminium; oxide, pages 133, 158-9, 269, GCSE Chemistry; Student Book, Collins, AQA'
- Aluminium; recycling, page 158, GCSE Chemistry; Student Book, Collins, AQA'