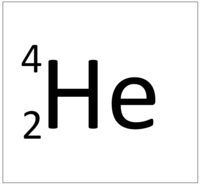Difference between revisions of "Atom"
(→Properties of the Subatomic Particles) |
|||
| Line 96: | Line 96: | ||
===History of Atoms=== | ===History of Atoms=== | ||
| + | ====Discovery of Atoms==== | ||
: The existence and structure of [[atom]]s was not always known. | : The existence and structure of [[atom]]s was not always known. | ||
| + | : An ancient Greek philosopher called [[Democratus]] first proposed that matter was made of [[atom]]s. He reasoned that if you keep cutting something in half eventually you will reach part of it which cannot be cut into smaller piece. The word for 'uncutable' was [[atom]]. | ||
| + | =====Dalton Model of the Atom===== | ||
| + | : It wasn't until the early 1800s that there was an [[Scientific Evidene|evidence]] based [[Scientific Theory|theory]] of the existence of an [[atom]]. It was proposed by [[John Dalton]] who suggested [[atom]]s were small [[sphere|spherical]] [[object]]s. | ||
| + | : [[John Dalton]] had his [[Scientific Theory|theory]] backed up by the discoveries of [[Robert Brown]] who discovered [[Brownian Motion]] showing that small invisible [[particle]]s were reponsible for the apparent random motion of small visible [[particle]]s such as [[pollen]]. | ||
| + | =====Plum Pudding Model of the Atom===== | ||
| + | : In 1897 [[J.J. Thompson]] discovered that there was a [[particle]] smaller than an [[atom]] which he named the [[electron]] after another [[scientist]] had [[hypothesis|hypothesised]] their existence. The [[electron]] was found to be around 1860 times less [[mass]]ive than a [[Hydrogen]] [[atom]]. It was later realised that these [[electron]]s were responsible for [[Electrical Current|electrical current]] in [[metal]]s. | ||
| + | : This led to the [[Plum Pudding Model]] of the [[atom]] in which [[atom]]s were believed to be a solid ball of [[positive]] charge with [[electron]]s stuck inside to give an overall [[neutral]] charge to the [[atom]]. | ||
| + | =====Nuclear Model of the Atom===== | ||
| + | : In 1909 [[Ernest Rutherford]] set two students to work on an experiment to probe the structure of the [[atom]] in the hope to determine if the [[Plum Pudding Model]] was correct. | ||
| + | : [[Ernest Rutherford|Rutherford's]] students [[Ernest Marsden]] and [[Hans Geiger]] fired [[Alpha Particle|alpha particle]]s, which are positively charged, at a very thin sheet of [[Gold]] foil to observe how the [[Alpha Particle|alpha particles]] changed direction as they went through the foil. | ||
| + | : They discovered that most of the [[Alpha Particle|alpha particle]]s went through in a straight line. A significant number were [[deflect]]ed and a very small number bounced off the [[Gold]] back towards the [[Alpha Particle|alpha]] source. | ||
| + | : If the [[Plum Pudding Model]] were correct then nearly all of the [[Alpha Particle|alpha particles]] should have passed straight through, unaffected, since the [[Alpha Particle|alpha particle]] is positively charged wile [[atom]]s should have an even spread of [[charge]]d [[particle]]s all the way through them. There should have been no [[Electrostatic Force|electrostatic force]] to change their direction. | ||
| + | : This showed that the [[atom]] must be mostly empty space, that most of the [[mass]] of an [[atom]] is concentrated in the centre and that the centre is positively charged. This gave rise to the [[Nuclear Model]] in which a very small positively charged [[Atomic Nucleus|nucleus]] is surrounded by [[orbit]]ing [[electron]]s. | ||
| + | =====The Bohr Model of the Atom===== | ||
Revision as of 14:37, 23 November 2018
Contents
Key Stage 3
Meaning
An atom is a very small particle made of protons, neutrons and electrons that can join with other atoms to make molecules.
About Atoms in The Dalton Model
- In The Dalton Model atoms are shown as ball shaped particles. This makes it easier to draw diagrams of molecules.
| A picture of The Dalton Model of an atom. |
About Atoms beyond The Dalton Model
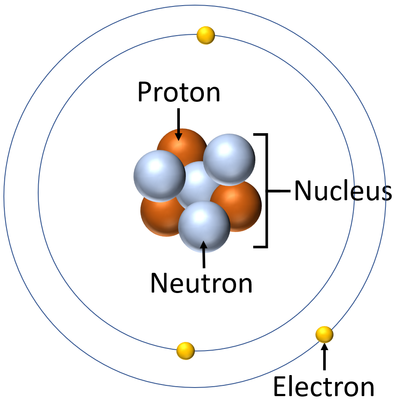
- Atoms are made of three smaller particles; the proton, neutron and electron.
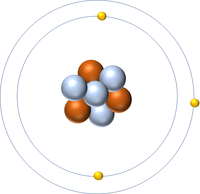
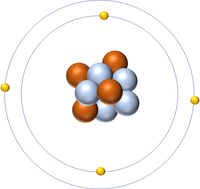
- Protons and neutrons are found in the nucleus at the centre of an atom. Electrons are found orbiting the nucleus in 'shells'.
| A diagram of an atom. |
- In an atom the number of electrons is always the same as the number of protons in the nucleus.
- Different atoms can have different numbers of protons and neutrons.
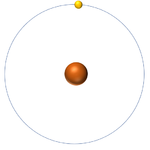
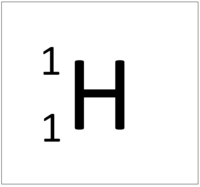
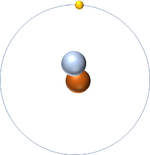
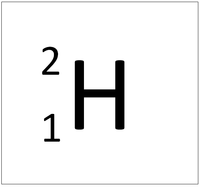
- The simplest atom is Hydrogen which has 1 proton and 1 electron and no neutrons.
Key Stage 4
Meaning
An atom is a very small particle made of protons, neutrons and electrons that can join with other atoms to make molecules.
About Atoms
- Atoms consist of a small, central nucleus containing protons and neutrons surrounded by electrons orbiting the nucleus.
- The electrons orbit the nucleus in so called 'electron shells.
| A diagram of an atom. |
- In an atom there is always the same number of protons as electrons. If any electron is added or removed the atom becomes an ion.
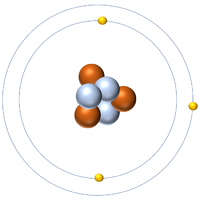
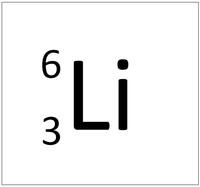
| Hydrogen | Helium | Lithium | Beryllium |
| Hydrogen always has 1 proton. | Helium always has 2 protons. | Lithium always has 3 protons. | Beryllium always has 4 protons. |
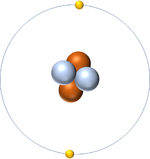
- Atoms of the same element can have different numbers of neutrons so they can be different isotopes of the same element.
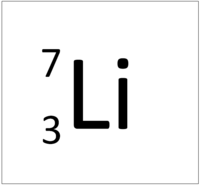
| Hydrogen-1 | Hydrogen-2 | Lithium-7 | Lithium-6 |
| Hydrogen always has 1 proton but in this case has no neutrons. | Hydrogen always has 1 proton but in this case also has a neutron. This isotope of Hydrogen is known as Deuterium. | Lithium always has 3 protons but in this case has 4 neutrons. | Lithium always has 3 protons but in this case has 3 neutrons. |
Properties of the Subatomic Particles
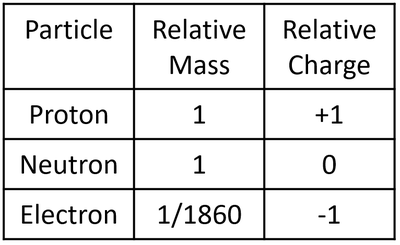
- The particles that make an atom all have slightly different properties. Since the particles are so small their mass and charge are extremely small numbers so, to make it easier, they are represented as 'relative' mass and 'relative' charge compared to a proton.
| A table showing the relative mass and relative charge of the proton, neutron and electron. |
History of Atoms
Discovery of Atoms
- The existence and structure of atoms was not always known.
- An ancient Greek philosopher called Democratus first proposed that matter was made of atoms. He reasoned that if you keep cutting something in half eventually you will reach part of it which cannot be cut into smaller piece. The word for 'uncutable' was atom.
Dalton Model of the Atom
- It wasn't until the early 1800s that there was an evidence based theory of the existence of an atom. It was proposed by John Dalton who suggested atoms were small spherical objects.
- John Dalton had his theory backed up by the discoveries of Robert Brown who discovered Brownian Motion showing that small invisible particles were reponsible for the apparent random motion of small visible particles such as pollen.
Plum Pudding Model of the Atom
- In 1897 J.J. Thompson discovered that there was a particle smaller than an atom which he named the electron after another scientist had hypothesised their existence. The electron was found to be around 1860 times less massive than a Hydrogen atom. It was later realised that these electrons were responsible for electrical current in metals.
- This led to the Plum Pudding Model of the atom in which atoms were believed to be a solid ball of positive charge with electrons stuck inside to give an overall neutral charge to the atom.
Nuclear Model of the Atom
- In 1909 Ernest Rutherford set two students to work on an experiment to probe the structure of the atom in the hope to determine if the Plum Pudding Model was correct.
- Rutherford's students Ernest Marsden and Hans Geiger fired alpha particles, which are positively charged, at a very thin sheet of Gold foil to observe how the alpha particles changed direction as they went through the foil.
- They discovered that most of the alpha particles went through in a straight line. A significant number were deflected and a very small number bounced off the Gold back towards the alpha source.
- If the Plum Pudding Model were correct then nearly all of the alpha particles should have passed straight through, unaffected, since the alpha particle is positively charged wile atoms should have an even spread of charged particles all the way through them. There should have been no electrostatic force to change their direction.
- This showed that the atom must be mostly empty space, that most of the mass of an atom is concentrated in the centre and that the centre is positively charged. This gave rise to the Nuclear Model in which a very small positively charged nucleus is surrounded by orbiting electrons.