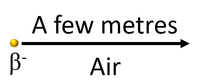Difference between revisions of "Beta Particle"
(→Precautions) |
|||
| Line 46: | Line 46: | ||
: When handling a source of [[Beta Particle|beta radiation]] the precautions which should be taken are: | : When handling a source of [[Beta Particle|beta radiation]] the precautions which should be taken are: | ||
:*Wear gloves - to prevent [[Radioactive Contamination|contamination]]. | :*Wear gloves - to prevent [[Radioactive Contamination|contamination]]. | ||
| − | :*Use tongs to handle the source, never touch it - to prevent [[Radioactive Contamination|contamination]]. | + | :*Use tongs to handle the source, never touch it - to prevent [[Radioactive Contamination|contamination]] and reduce [[irradiation]] (the tongs won't be long enough to prevent [[irradiation]].) |
:*Stand behind a [[metal]] screen - to prevent [[irradiation]]. | :*Stand behind a [[metal]] screen - to prevent [[irradiation]]. | ||
:*Aim the source away from any living [[organism]] - to prevent [[irradiation]]. | :*Aim the source away from any living [[organism]] - to prevent [[irradiation]]. | ||
Revision as of 09:18, 8 March 2019
Contents
Key Stage 4
Meaning
An beta particle (β-particle) is a type of ionising radiation made of an electron emitted from the nucleus of an unstable isotope when a neutron turns into a proton.
About Beta Particles
- Beta particles may also be referred to as beta radiation and is written with the symbol β.
- Beta particles are a fast moving electron emitted from the nucleus of an unstable isotope.
- Beta particles have a relative atomic mass of 1/2000 (which is so much smaller than a nucleon that it is usually referred to as 0) and relative charge of -1.
- Beta particles are emitted when a nucleus is too large or the ratio of neutrons to protons is too large (too many neutrons).
Charge and Mass
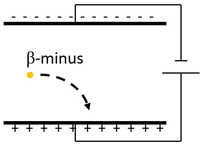
| Scientist were able to determine the charge and mass of a β-particle by sending it between two electrically charged plates and observing its path.
The β-particle moves towards the positive plate, so it must be positively charged. The rate of curvature depends on the mass:charge ratio which indicates it has a relative atomic mass of 1/2000 and relative charge of -1. |
Penetration Depth
| Beta particles can travel several metres through air (STP) before colliding with and ionising atoms or molecules. |
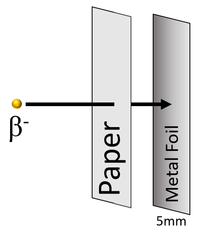
| Beta particles can penetrate paper but are stopped by around 5mm thickness of metal foil. |
Ionising Potential
- With a charge of -1, β-particles are the second most ionising of the three ionising radiations. It is capable of knocking out more than one electron from different atoms or molecules as it has a large amount of kinetic energy.
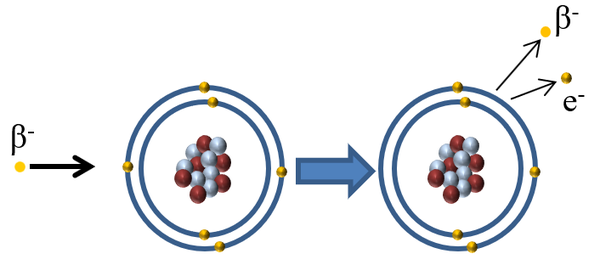
| When a beta particle interacts with an atom the beta minus particle can pass on some of its kinetic energy to an electron in the outer shell causing the electron to escape ionising the atom. This may happen more than once if the beta particle collides with another atom with enough energy. |
Precautions
- Beta radiation is the second most ionising and the second most penetrating.
- Beta sources are kept inside a block of lead with a hole that only allows the beta particles out in one direction.
- Outside the body an organism cannot easily be protected from beta radiation as it travels several metres through the air and needs a metal barrier to block it. beta radiation can pass through the skin and ionise tissue deep within the body.
- When handling a source of beta radiation the precautions which should be taken are:
- Wear gloves - to prevent contamination.
- Use tongs to handle the source, never touch it - to prevent contamination and reduce irradiation (the tongs won't be long enough to prevent irradiation.)
- Stand behind a metal screen - to prevent irradiation.
- Aim the source away from any living organism - to prevent irradiation.
- Store the source in a sealed container - to prevent contamination and irradiation.
Applications
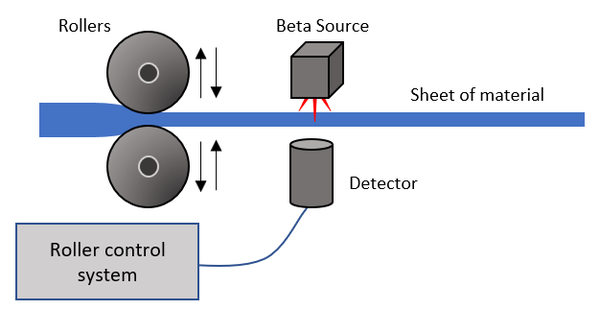
| Beta particles can be used to detect the thickness of a material. The thicker the material the less Beta particles will make it to the detector.
When manufacturing sheets of material a beta source and detector can be used to control the separation of a pair of rollers. When the material becomes too thick the rollers move closer together, squashing the material. When the material becomes to thin the rollers move further apart to squash the material less. |