Difference between revisions of "Lithium"
(→Atomic Structure) |
|||
| Line 31: | Line 31: | ||
: An [[atom]] of [[Lithium]] has only 1 [[electron]] in its [[Outer Shell|outer shell]]. | : An [[atom]] of [[Lithium]] has only 1 [[electron]] in its [[Outer Shell|outer shell]]. | ||
: [[Lithium]] [[ion]]s have lost an [[electron]] to become [[Positive Charged|positively charged]]. | : [[Lithium]] [[ion]]s have lost an [[electron]] to become [[Positive Charged|positively charged]]. | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:LithiumIonFormation.png|center|400px]] | ||
| + | |- | ||
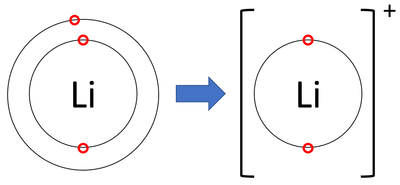
| + | | style="height:20px; width:200px; text-align:center;" |A [[diagram]] showing the formation of a [[Lithium]] [[ion]]. | ||
| + | |} | ||
| + | |||
====Properties==== | ====Properties==== | ||
: [[Lithium]] is the least [[Reactivity|reactive]] [[Alkali Metal]]. | : [[Lithium]] is the least [[Reactivity|reactive]] [[Alkali Metal]]. | ||
Revision as of 19:57, 31 March 2019
Contents
Key Stage 2
Meaning
Key Stage 3
Meaning
Lithium is a Group 1 element, on the Periodic Table, with an atomic number of 3.
About Lithium
Molecular Structure
- Lithium has the chemical formula Li.
- Lithium atoms join together in large numbers to form a giant metal molecule.
Atomic Structure
- Lithium has 3 protons and 4 neutrons in its nucleus giving it an Atomic Number of 3 and an atomic mass of 7.
- An atom of Lithium has only 1 electron in its outer shell.
Properties
- Lithium is the least reactive Alkali Metal.
- Lithium is more reactive than Carbon on the reactivity series so it must be extracted from its ore using electrolysis.
- Lithium oxidises quickly in the presence of Oxygen so it must be stored in oil.
- Lithium reacts strongly with water to produce Hydrogen gas and Lithium Hydroxide.
- Lithium is a solid at room temperature.
Key Stage 4
Meaning
Lithium is a Group 1 element, on the Periodic Table, with 3 protons in the nucleus.
About Lithium
Molecular Structure
- Lithium has the chemical formula Li.
- Lithium atoms join together in a giant metallic structure.
Atomic Structure
- The most stable isotope of Lithium has 4 neutrons in its nucleus giving it an atomic mass of 7.
- An atom of Lithium has only 1 electron in its outer shell.
- Lithium ions have lost an electron to become positively charged.
| A diagram showing the formation of a Lithium ion. |
Properties
- Lithium is the least reactive Alkali Metal.
- Lithium is more reactive than Carbon on the reactivity series so it must be extracted from its ore using electrolysis.
- Lithium oxidises quickly in the presence of Oxygen so it must be stored in oil.
- Lithium reacts strongly with water to produce Hydrogen gas and Lithium Hydroxide.
- Lithium is a solid at standard temperature and pressure with a melting point of 180.50 °C.