Difference between revisions of "Transition Metal"
| (19 intermediate revisions by 2 users not shown) | |||
| Line 4: | Line 4: | ||
===About Transition Metals=== | ===About Transition Metals=== | ||
| + | : [[Transition Metal]]s have the [[Physical Property|physical properties]] of [[metal]]s. | ||
| + | : [[Transition Metal]]s [[Chemical Bond|bond]] together with [[Metallic Bond|metallic bonds]] in which [[Positive Ion|positive ions]] are surrounded by a sea of [[Negative Charge|negatively charged]] [[electron]]s (known as [[Delocalised Electrons|delocalised electrons]]. | ||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
| Line 9: | Line 11: | ||
|- style="background:{{element color|table header}}" | | |- style="background:{{element color|table header}}" | | ||
|- | |- | ||
| − | |[[ | + | |[[Period 4]] |
|<sub>21</sub>[[Scandium|Sc]] | |<sub>21</sub>[[Scandium|Sc]] | ||
|<sub>22</sub>[[Titanium|Ti]] | |<sub>22</sub>[[Titanium|Ti]] | ||
| Line 21: | Line 23: | ||
|<sub>30</sub>[[Zinc|Zn]] | |<sub>30</sub>[[Zinc|Zn]] | ||
|- | |- | ||
| − | |[[ | + | |[[Period 5]] |
|<sub>39</sub>[[Yttrium|Y]] | |<sub>39</sub>[[Yttrium|Y]] | ||
|<sub>40</sub>[[Zirconium|Zr]] | |<sub>40</sub>[[Zirconium|Zr]] | ||
| Line 33: | Line 35: | ||
|<sub>48</sub>[[Cadmium|Cd]] | |<sub>48</sub>[[Cadmium|Cd]] | ||
|- | |- | ||
| − | | [[ | + | | [[Period 6]] |
|<sub>57</sub>[[Lanthanum|La]] | |<sub>57</sub>[[Lanthanum|La]] | ||
|<sub>72</sub>[[Hafnium|Hf]] | |<sub>72</sub>[[Hafnium|Hf]] | ||
| Line 43: | Line 45: | ||
|<sub>78</sub>[[Platinum|Pt]] | |<sub>78</sub>[[Platinum|Pt]] | ||
|<sub>79</sub>[[Gold|Au]] | |<sub>79</sub>[[Gold|Au]] | ||
| − | | <sub>80</sub>[[Mercury ( | + | | <sub>80</sub>[[Mercury (Element)|Hg]] |
|- | |- | ||
| − | | [[ | + | | [[Period 7]] |
|<sub>89</sub>[[Actinium|Ac]] | |<sub>89</sub>[[Actinium|Ac]] | ||
| − | |<sub>104</sub> | + | |<sub>104</sub>Rf |
| − | |<sub>105</sub> | + | |<sub>105</sub>Db |
| − | |<sub>106</sub> | + | |<sub>106</sub>Sg |
| − | |<sub>107</sub> | + | |<sub>107</sub>Bh |
| − | |<sub>108</sub> | + | |<sub>108</sub>Hs |
| − | |<sub>109</sub> | + | |<sub>109</sub>Mt |
| − | |<sub>110</sub> | + | |<sub>110</sub>Ds |
| − | |<sub>111</sub> | + | |<sub>111</sub>Rg |
| − | |<sub>112</sub> | + | |<sub>112</sub>Cn |
|} | |} | ||
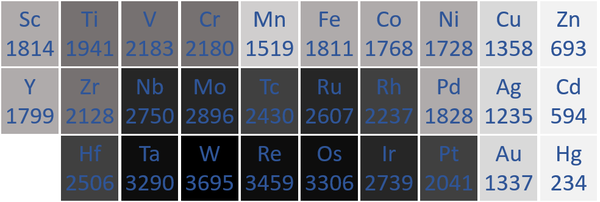
====Melting Point==== | ====Melting Point==== | ||
| − | : [[Transition Metal]]s usually have high [[Melting Point|melting points]]. | + | : [[Transition Metal]]s usually have high [[Melting Point|melting points]] because [[Metallic Bond|metallic bonds]] are very strong, keeping the [[atom]]s [[Vibration|vibrating]] in fixed positions. |
{| class="wikitable" | {| class="wikitable" | ||
| + | | style="height:20px; width:600px; text-align:center;" |The '''transition metal''' [[Melting Point|melting points]] measured in [[Kelvin]] are written below each [[Chemical Symbol|chemical symbol]]. | ||
|- | |- | ||
| − | |[[File:TransitionMetalMeltingPoints.png|center| | + | |[[File:TransitionMetalMeltingPoints.png|center|600px]] |
|- | |- | ||
| − | | style="height:20px; width: | + | | style="height:20px; width:600px; text-align:center;" |N.B. The [[Period 7]] [[element]]s have not been included as they do not occur naturally and have not been made in large enough quantities to find their [[Melting Point|melting points]]. |
|} | |} | ||
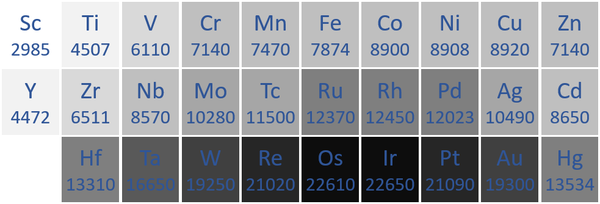
====Density==== | ====Density==== | ||
| − | : [[Transition Metal]]s have a high [[density]] compared to other [[element]]s. However, the | + | : [[Transition Metal]]s have a high [[density]] compared to other [[element]]s. However, many of the [[Actinide]]s also have a [[density]]. |
| − | ==== | + | {| class="wikitable" |
| + | | style="height:20px; width:600px; text-align:center;" |The '''transition metal''' [[Density|densities]] measured in [[Kilograms per Metre Cubed|kilograms per metre cubed]] are written below each [[Chemical Symbol|chemical symbol]]. | ||
| + | |- | ||
| + | |[[File:TransitionMetalDensity.png|center|600px]] | ||
| + | |- | ||
| + | | style="height:20px; width:600px; text-align:center;" |N.B. The [[Period 7]] [[element]]s have not been included as they do not occur naturally and have not been made in large enough quantities to find their [density|densities]]. | ||
| + | |} | ||
| + | |||
| + | ====Reactivity==== | ||
| + | : The [[Transition Metal]]s are much less [[Reactivity|reactive]] than [[element]]s in [[Group 1|group 1]] and [[Group 2|group 2]]. | ||
| + | : Most of the [[Transition Metal]]s [[Chemical Reaction|react]] slowly with [[Oxygen]], [[Water]] and the [[Halogen]]s at [[Room Temperature|room temperature]] and some will not [[Chemical Reaction|react]] at all. | ||
| + | : For [[combustion]] to occur the [[Transition Metal]]s must be heated to very high [[temperature]]s. However, some [[Transition Metal]]s will not [[combustion|combust]] at all. | ||
| + | |||
====Ion Formation==== | ====Ion Formation==== | ||
| − | ( | + | : [[Transition Metal]]s form [[Positive Ion|positive ions]] during [[Chemical Reaction|chemical reactions]]. |
| + | : [[Transition Metal]]s can form several different [[Electrical Charge|charge]] [[ion]]s and these different [[Electrical Charge|charge]]s can determine the [[Physical Property|physical properties]] of the [[compound]]s they are in. Specifically this affects the [[colour]] of those [[compound]]s. | ||
| + | In [[compound]]s the [[Electrical Charge|charge]] of the [[Transition Metal]] [[ion]] is given in Roman Numerals after the name of the [[metal]] | ||
| + | *[[Copper (I) Sulphate]]: +1 [[Ion]] of [[Copper]] giving the [[compound]] a light green colour. | ||
| + | *[[Copper (II) Sulphate]]: +2 [[Ion]] of [[Copper]] giving the [[compound]] a blue colour. | ||
| + | *[[Manganese (II) Chloride]]: +2 [[Ion]] of [[Manganese]] giving the [[compound]] a pale pink colour. | ||
| + | *[[Manganese (IV) Oxide]]: +4 [[Ion]] of [[Manganese]] giving it a very dark brown colour, often appearing black. | ||
| + | *[[Iron (II) Sulphate]]: *2 [[Ion]] of [[Iron]] giving the [[compound]] a pale green colour. | ||
| + | *[[Iron (III) Sulphate]]: *3 [[Ion]] of [[Iron]] giving the [[compound]] a redish-brown colour. | ||
| + | : Gem stones with various colours (red rubies, green emeralds, blue sapphires) get their colour from the [[Transition Metal]] [[ion]]s they contain. | ||
| + | |||
====Catalysts==== | ====Catalysts==== | ||
| + | : Many [[Transition Metal]]s can be used as [[catalyst]]s for [[Chemical Reaction|chemical reactions]]. | ||
| + | : [[Catalyst]]s can increase the rate of [[Chemical Reaction|reaction]] and lower the [[temperature]] needed to start a [[Chemical Reaction|reaction]]. | ||
| + | : [[Catalyst]]s are not used up in the [[Chemical Reaction|chemical reaction]] so can be used over and over again. | ||
| + | : [[Transition Metal]] [[catalyst]]s can increase the rate of [[Chemical Reaction|reaction]] by either accepting or donating [[electron]]s so that not as much [[energy]] is needed for a [[Chemical Reaction|reaction]] to happen. | ||
| + | These are some [[Transition Metal]] [[catalyst]]s you may know: | ||
| + | *[[Iron]]: Used as a [[catalyst]] for [[Hydrogen]] and [[Nitrogen]] reacting to produce [[Ammonia]]. | ||
| + | *[[Platinum]]: Used in the production of [[Nitric Acid]]. | ||
| + | *[[Manganese]]: Used in the form of [[Manganese Dioxide]] as a [[catalyst]] for the [[Decomposition Reaction|decomposition]] of [[Hydrogen Peroxide]]. | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Transition metal, pages 48-9, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851346/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851346&linkCode=as2&tag=nrjc-21&linkId=3ac654f4b0da781c49c855a1af4c92ea ''Transition metals, pages 18-19, GCSE Chemistry, Hodder, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945571/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945571&linkCode=as2&tag=nrjc-21&linkId=9e29fad914244909903e5e93f8a01d261 ''Transition metals, pages 23, 24, 25, GCSE Chemistry; The Revision Guide, CGP, AQA ''] | ||
| + | |||
| + | ====Edexcel==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/1782945725/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945725&linkCode=as2&tag=nrjc-21&linkId=694be7494de75af3349537d34e13f7f0 ''Transition metals, page 62, GCSE Chemistry; The Revision Guide, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1782948147/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782948147&linkCode=as2&tag=nrjc-21&linkId=f63dcd8345f4e49c717b39a228a36c7c ''Transition metals, pages 177, 178, GCSE Chemistry, CGP, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Transition metals, pages 96-97, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Transition metals; chemical properties, page 97, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1292120215/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1292120215&linkCode=as2&tag=nrjc-21&linkId=8f96ddb76196848bafdb124354e4cf77 ''Transition metals; physical properties, pages 96-97, GCSE Chemistry, Pearson, Edexcel ''] | ||
| + | |||
| + | ====OCR==== | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945679/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945679&linkCode=as2&tag=nrjc-21&linkId=a2db42f7b4bdf10cafaafa3bb9120940 ''Transition metals, page 55, Gateway GCSE Chemistry; The Revision Guide, CGP, OCR ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0198359829/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359829&linkCode=as2&tag=nrjc-21&linkId=90e8d7b4f039d53035238fa0320fe00b ''Transition metals, pages 88, 140-141, Gateway GCSE Chemistry, Oxford, OCR ''] | ||
Latest revision as of 17:26, 20 December 2019
Contents
Key Stage 4
Meaning
Transition Metals (also known as transition elements) are a block of elements on the Periodic Table between Group 2 and Group 3.
About Transition Metals
- Transition Metals have the physical properties of metals.
- Transition Metals bond together with metallic bonds in which positive ions are surrounded by a sea of negatively charged electrons (known as delocalised electrons.
| Period 4 | 21Sc | 22Ti | 23V | 24Cr | 25Mn | 26Fe | 27Co | 28Ni | 29Cu | 30Zn |
| Period 5 | 39Y | 40Zr | 41Nb | 42Mo | 43Tc | 44Ru | 45Rh | 46Pd | 47Ag | 48Cd |
| Period 6 | 57La | 72Hf | 73Ta | 74W | 75Re | 76Os | 77Ir | 78Pt | 79Au | 80Hg |
| Period 7 | 89Ac | 104Rf | 105Db | 106Sg | 107Bh | 108Hs | 109Mt | 110Ds | 111Rg | 112Cn |
Melting Point
- Transition Metals usually have high melting points because metallic bonds are very strong, keeping the atoms vibrating in fixed positions.
| The transition metal melting points measured in Kelvin are written below each chemical symbol. |
| N.B. The Period 7 elements have not been included as they do not occur naturally and have not been made in large enough quantities to find their melting points. |
Density
- Transition Metals have a high density compared to other elements. However, many of the Actinides also have a density.
| The transition metal densities measured in kilograms per metre cubed are written below each chemical symbol. |
| N.B. The Period 7 elements have not been included as they do not occur naturally and have not been made in large enough quantities to find their [density|densities]]. |
Reactivity
- The Transition Metals are much less reactive than elements in group 1 and group 2.
- Most of the Transition Metals react slowly with Oxygen, Water and the Halogens at room temperature and some will not react at all.
- For combustion to occur the Transition Metals must be heated to very high temperatures. However, some Transition Metals will not combust at all.
Ion Formation
- Transition Metals form positive ions during chemical reactions.
- Transition Metals can form several different charge ions and these different charges can determine the physical properties of the compounds they are in. Specifically this affects the colour of those compounds.
In compounds the charge of the Transition Metal ion is given in Roman Numerals after the name of the metal
- Copper (I) Sulphate: +1 Ion of Copper giving the compound a light green colour.
- Copper (II) Sulphate: +2 Ion of Copper giving the compound a blue colour.
- Manganese (II) Chloride: +2 Ion of Manganese giving the compound a pale pink colour.
- Manganese (IV) Oxide: +4 Ion of Manganese giving it a very dark brown colour, often appearing black.
- Iron (II) Sulphate: *2 Ion of Iron giving the compound a pale green colour.
- Iron (III) Sulphate: *3 Ion of Iron giving the compound a redish-brown colour.
- Gem stones with various colours (red rubies, green emeralds, blue sapphires) get their colour from the Transition Metal ions they contain.
Catalysts
- Many Transition Metals can be used as catalysts for chemical reactions.
- Catalysts can increase the rate of reaction and lower the temperature needed to start a reaction.
- Catalysts are not used up in the chemical reaction so can be used over and over again.
- Transition Metal catalysts can increase the rate of reaction by either accepting or donating electrons so that not as much energy is needed for a reaction to happen.
These are some Transition Metal catalysts you may know:
- Iron: Used as a catalyst for Hydrogen and Nitrogen reacting to produce Ammonia.
- Platinum: Used in the production of Nitric Acid.
- Manganese: Used in the form of Manganese Dioxide as a catalyst for the decomposition of Hydrogen Peroxide.
References
AQA
- Transition metal, pages 48-9, GCSE Chemistry; Student Book, Collins, AQA
- Transition metals, pages 18-19, GCSE Chemistry, Hodder, AQA
- Transition metals, pages 23, 24, 25, GCSE Chemistry; The Revision Guide, CGP, AQA
Edexcel
- Transition metals, page 62, GCSE Chemistry; The Revision Guide, CGP, Edexcel
- Transition metals, pages 177, 178, GCSE Chemistry, CGP, Edexcel
- Transition metals, pages 96-97, GCSE Chemistry, Pearson, Edexcel
- Transition metals; chemical properties, page 97, GCSE Chemistry, Pearson, Edexcel
- Transition metals; physical properties, pages 96-97, GCSE Chemistry, Pearson, Edexcel