Difference between revisions of "Bromine"
(Created page with "==Key Stage 2== ===Meaning=== Bromine is a brown liquid. ==Key Stage 3== ===Meaning=== File:BromineSymbol.png|right|300px|thumb|The [[Chemical Symbol|chemical symbol...") |
|||
| (15 intermediate revisions by the same user not shown) | |||
| Line 4: | Line 4: | ||
==Key Stage 3== | ==Key Stage 3== | ||
===Meaning=== | ===Meaning=== | ||
| − | [[File: | + | [[File:BromineSymbol1.png|right|300px|thumb|The [[Chemical Symbol|chemical symbol]] for [[Bromine]].]] |
[[Bromine]] is a [[Group 7]] [[element]], on the [[Periodic Table]], with an [[Atomic Number|atomic number]] of 35. | [[Bromine]] is a [[Group 7]] [[element]], on the [[Periodic Table]], with an [[Atomic Number|atomic number]] of 35. | ||
===About Bromine=== | ===About Bromine=== | ||
| + | ====Molecular Structure==== | ||
: [[Bromine]] has the [[Chemical Formula|chemical formula]] [[Bromine| Br<sub>2</sub>]]. | : [[Bromine]] has the [[Chemical Formula|chemical formula]] [[Bromine| Br<sub>2</sub>]]. | ||
| + | ====Atomic Structure==== | ||
: [[Bromine]] as 35 [[proton]]s and 45 [[neutron]]s in its [[Atomic Nucleus|nucleus]] giving it an [[Atomic Number]] of 35 and an [[Relative Atomic Mass|atomic mass]] of 80. | : [[Bromine]] as 35 [[proton]]s and 45 [[neutron]]s in its [[Atomic Nucleus|nucleus]] giving it an [[Atomic Number]] of 35 and an [[Relative Atomic Mass|atomic mass]] of 80. | ||
| + | : An [[atom]] of [[Bromine]] is missing one [[electron]] from having a full [[Outer Shell|outer shell]]. | ||
| + | |||
| + | ====Properties==== | ||
| + | : [[Bromine]] is a [[non-metal]] [[element]]. | ||
: [[Bromine]] is a more [[Reactivity|reactive]] [[Halogen]] than [[Iodine]] but less [[Reactivity|reactive]] than [[Chlorine]]. | : [[Bromine]] is a more [[Reactivity|reactive]] [[Halogen]] than [[Iodine]] but less [[Reactivity|reactive]] than [[Chlorine]]. | ||
| − | : [[Bromine]] [[Chemical Reaction|reacts]] strongly with [[Hydrogen]] to produce [[Hydrogen | + | : [[Bromine]] [[Chemical Reaction|reacts]] strongly with [[Hydrogen]] to produce [[Hydrogen Bromide]] which [[dissolve]]s in [[water]] to produce [[Hydrobromic Acid]]. |
: [[Bromine]] is a [[bleach]]ing agent. | : [[Bromine]] is a [[bleach]]ing agent. | ||
: [[Bromine]] kills [[bacteria]]. | : [[Bromine]] kills [[bacteria]]. | ||
: [[Bromine]] is a pale green coloured [[gas]] at [[STP|room temperature]]. | : [[Bromine]] is a pale green coloured [[gas]] at [[STP|room temperature]]. | ||
| − | + | ||
| − | |||
==Key Stage 4== | ==Key Stage 4== | ||
| + | [[File:BrKS4.PNG|right|200px|thumb|The [[Chemical Symbol|chemical symbol]] for [[Bromine]].]] | ||
===Meaning=== | ===Meaning=== | ||
| − | [[Bromine]] is a [[Group | + | [[Bromine]] is a [[Group 7]] [[element]], on the [[Periodic Table]], with 35 [[proton]]s in the [[Atomic Nucleus|nucleus]]. |
===About Bromine=== | ===About Bromine=== | ||
| + | ====Molecular Structure==== | ||
: [[Bromine]] has the [[Chemical Formula|chemical formula]] [[Bromine|Br<sub>2</sub>]]. | : [[Bromine]] has the [[Chemical Formula|chemical formula]] [[Bromine|Br<sub>2</sub>]]. | ||
| + | : [[Bromine]] [[atom]]s join together in a [[Covalent Bond|covalent bond]]. | ||
| + | {| class="wikitable" | ||
| + | |- | ||
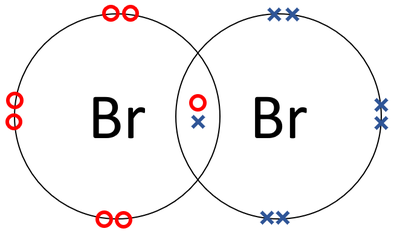
| + | |[[File:BromineDotandCrossDiagram.png|center|400px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |A [[Dot and Cross Diagram|dot and cross diagram]] of a [[Bromine]] [[molecule]]. | ||
| + | |} | ||
| + | ====Atomic Structure==== | ||
: The most [[Stable Isotope|stable isotope]] of [[Bromine]] has 45 [[neutron]]s in its [[Atomic Nucleus|nucleus]] giving it an [[Relative Atomic Mass|atomic mass]] of 80. | : The most [[Stable Isotope|stable isotope]] of [[Bromine]] has 45 [[neutron]]s in its [[Atomic Nucleus|nucleus]] giving it an [[Relative Atomic Mass|atomic mass]] of 80. | ||
| + | : An [[atom]] of [[Bromine]] is missing one [[electron]] from having a full [[Outer Shell|outer shell]]. | ||
| + | : [[Bromine]] [[ion]]s gain 1 [[electron]] to get a full [[Outer Shell|outer shell]] and become [[Negative Charge|negatively charged]]. | ||
| + | ====Properties==== | ||
| + | : [[Bromine]] is a [[non-metal]] [[element]]. | ||
: [[Bromine]] is a more [[Reactivity|reactive]] [[Halogen]] than [[Iodine]] but less [[Reactivity|reactive]] than [[Chlorine]]. | : [[Bromine]] is a more [[Reactivity|reactive]] [[Halogen]] than [[Iodine]] but less [[Reactivity|reactive]] than [[Chlorine]]. | ||
: [[Bromine]] [[Chemical Reaction|reacts]] strongly with [[Hydrogen]] to produce [[Hydrogen Bromide]] which [[dissolve]]s in [[water]] to produce [[Hydrobromic Acid]]. | : [[Bromine]] [[Chemical Reaction|reacts]] strongly with [[Hydrogen]] to produce [[Hydrogen Bromide]] which [[dissolve]]s in [[water]] to produce [[Hydrobromic Acid]]. | ||
| Line 27: | Line 46: | ||
: [[Bromine]] kills [[bacteria]]. | : [[Bromine]] kills [[bacteria]]. | ||
: [[Bromine]] is a brown coloured [[gas]] at [[STP|standard temperature and pressure]]. | : [[Bromine]] is a brown coloured [[gas]] at [[STP|standard temperature and pressure]]. | ||
| − | : | + | |
| − | : [ | + | ===References=== |
| + | ====AQA==== | ||
| + | :[https://www.amazon.co.uk/gp/product/1782945962/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1782945962&linkCode=as2&tag=nrjc-21&linkId=476bb5c8d1dfb5c08ac81b6d4d1c98d8 ''Bromine, page 61, GCSE Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/178294639X/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=178294639X&linkCode=as2&tag=nrjc-21&linkId=51599bb45a2bfaf7c1b6a978b2ca2616 ''Bromine, page 61, GCSE Combined Science Trilogy; Chemistry, CGP, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/1471851362/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=1471851362&linkCode=as2&tag=nrjc-21&linkId=7d78d70a2044ee9982dae010c94af92a ''Bromine, reaction with alkenes, pages 148, GCSE Combined Science Trilogy 2, Hodder, AQA ''] | ||
Latest revision as of 11:17, 5 March 2020
Contents
Key Stage 2
Meaning
Key Stage 3
Meaning
Bromine is a Group 7 element, on the Periodic Table, with an atomic number of 35.
About Bromine
Molecular Structure
- Bromine has the chemical formula Br2.
Atomic Structure
- Bromine as 35 protons and 45 neutrons in its nucleus giving it an Atomic Number of 35 and an atomic mass of 80.
- An atom of Bromine is missing one electron from having a full outer shell.
Properties
- Bromine is a non-metal element.
- Bromine is a more reactive Halogen than Iodine but less reactive than Chlorine.
- Bromine reacts strongly with Hydrogen to produce Hydrogen Bromide which dissolves in water to produce Hydrobromic Acid.
- Bromine is a bleaching agent.
- Bromine kills bacteria.
- Bromine is a pale green coloured gas at room temperature.
Key Stage 4
Meaning
Bromine is a Group 7 element, on the Periodic Table, with 35 protons in the nucleus.
About Bromine
Molecular Structure
- Bromine has the chemical formula Br2.
- Bromine atoms join together in a covalent bond.
| A dot and cross diagram of a Bromine molecule. |
Atomic Structure
- The most stable isotope of Bromine has 45 neutrons in its nucleus giving it an atomic mass of 80.
- An atom of Bromine is missing one electron from having a full outer shell.
- Bromine ions gain 1 electron to get a full outer shell and become negatively charged.
Properties
- Bromine is a non-metal element.
- Bromine is a more reactive Halogen than Iodine but less reactive than Chlorine.
- Bromine reacts strongly with Hydrogen to produce Hydrogen Bromide which dissolves in water to produce Hydrobromic Acid.
- Bromine is a bleaching agent.
- Bromine kills bacteria.
- Bromine is a brown coloured gas at standard temperature and pressure.