Contents
Key Stage 4
Meaning
Transition Metals (also known as transition elements) are a block of elements on the Periodic Table between Group 2 and Group 3.
About Transition Metals
- Transition Metals have the physical properties of metals.
- Transition Metals bond together with metallic bonds in which positive ions are surrounded by a sea of negatively charged electrons (known as delocalised electrons.
| Period 4 | 21Sc | 22Ti | 23V | 24Cr | 25Mn | 26Fe | 27Co | 28Ni | 29Cu | 30Zn |
| Period 5 | 39Y | 40Zr | 41Nb | 42Mo | 43Tc | 44Ru | 45Rh | 46Pd | 47Ag | 48Cd |
| Period 6 | 57La | 72Hf | 73Ta | 74W | 75Re | 76Os | 77Ir | 78Pt | 79Au | 80Hg |
| Period 7 | 89Ac | 104Rf | 105Db | 106Sg | 107Bh | 108Hs | 109Mt | 110Ds | 111Rg | 112Cn |
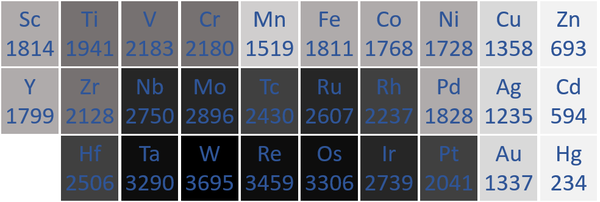
Melting Point
- Transition Metals usually have high melting points because metallic bonds are very strong, keeping the atoms vibrating in fixed positions.
| The transition metal melting points measured in Kelvin are written below each chemical symbol. |
| N.B. The Period 7 elements have not been included as they do not occur naturally and have not been made in large enough quantities to find their melting points. |
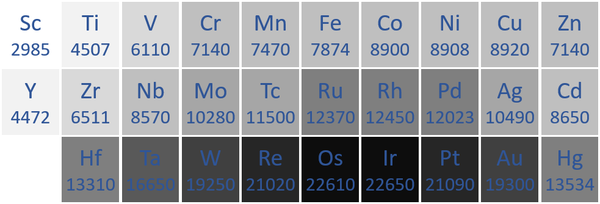
Density
- Transition Metals have a high density compared to other elements. However, many of the Actinides also have a density.
| The transition metal densities measured in kilograms per metre cubed are written below each chemical symbol. |
| N.B. The Period 7 elements have not been included as they do not occur naturally and have not been made in large enough quantities to find their [density|densities]]. |
Reactivity
- The Transition Metals are much less reactive than elements in group 1 and group 2.
- Most of the Transition Metals react slowly with Oxygen, Water and the Halogens at room temperature and some will not react at all.
- For combustion to occur the Transition Metals must be heated to very high temperatures. However, some Transition Metals will not combust at all.
Ion Formation
- Transition Metals form positive ions during chemical reactions.