Difference between revisions of "Potassium"
| Line 37: | Line 37: | ||
: [[Potassium]] [[Chemical Reaction|reacts]] strongly with [[water]] to produce [[Hydrogen]] [[gas]] and [[Potassium Hydroxide]]. | : [[Potassium]] [[Chemical Reaction|reacts]] strongly with [[water]] to produce [[Hydrogen]] [[gas]] and [[Potassium Hydroxide]]. | ||
: [[Potassium]] is a [[solid]] at [[STP|standard temperature and pressure]] with a [[Melting Point|melting point]] of 63.5 [[Degrees Celsius|°C]]. | : [[Potassium]] is a [[solid]] at [[STP|standard temperature and pressure]] with a [[Melting Point|melting point]] of 63.5 [[Degrees Celsius|°C]]. | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Potassium, pages 42-4, 134, 275, 285, 346, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Potassium; chloride, pages 44, 71, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Potassium; dichromate (VI), page 243, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Potassium; hydroxide, pages 148-9, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Potassium; iodide, page 115, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Potassium; oxide, page 35, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158762/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158762&linkCode=as2&tag=nrjc-21&linkId=a0fffa35b3ea49a63404f6704e0df7cc ''Potassium; sulphide, page 91, GCSE Chemistry; Student Book, Collins, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158770/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158770&linkCode=as2&tag=nrjc-21&linkId=ec31595e720e1529e49876c3866fff6e ''Potassium-40, page 122, GCSE Physics; Student Book, Collins, AQA ''] | ||
Revision as of 22:07, 10 November 2019
Contents
Key Stage 2
Meaning
Key Stage 3
Meaning
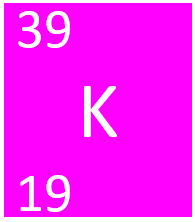
Potassium is a Group 1 element, on the Periodic Table, with an atomic number of 19.
About Potassium
Molecular Structure
- Potassium has the chemical symbol K.
- Potassium atoms join together in large numbers to form a giant metal molecule.
Atomic Structure
- Potassium has 19 protons and 20 neutrons in its nucleus giving it an Atomic Number of 19 and an atomic mass of 39.
- An atom of Potassium has only 1 electron in its outer shell.
Properties
- Potassium is a more reactive alkali metal than Sodium but less reactive than Rubidium.
- Potassium is more reactive than Carbon on the reactivity series so it must be extracted from its ore using electrolysis.
- Potassium oxidises quickly in the presence of Oxygen so it must be stored in oil.
- Potassium reacts strongly with water to produce Hydrogen gas and Potassium Hydroxide.
- Potassium is a solid at room temperature.
Key Stage 4
Meaning
Potassium is a Group 1 element, on the Periodic Table, with 19 protons in the nucleus.
About Potassium
Molecular Structure
- Potassium has the chemical symbol K.
- Potassium atoms join together in a giant metallic structure.
Atomic Structure
- The most stable isotope of Potassium has 20 neutrons in its nucleus giving it an atomic mass of 39.
- An atom of Potassium has only 1 electron in its outer shell.
- Potassium ions have lost an electron to become positively charged.
Properties
- Potassium is a more reactive alkali metal than Sodium but less reactive than Rubidium.
- Potassium is more reactive than Carbon on the reactivity series so it must be extracted from its ore using electrolysis.
- Potassium oxidises quickly in the presence of Oxygen so it must be stored in oil.
- Potassium reacts strongly with water to produce Hydrogen gas and Potassium Hydroxide.
- Potassium is a solid at standard temperature and pressure with a melting point of 63.5 °C.
References
AQA
- Potassium, pages 42-4, 134, 275, 285, 346, GCSE Chemistry; Student Book, Collins, AQA
- Potassium; chloride, pages 44, 71, GCSE Chemistry; Student Book, Collins, AQA
- Potassium; dichromate (VI), page 243, GCSE Chemistry; Student Book, Collins, AQA
- Potassium; hydroxide, pages 148-9, GCSE Chemistry; Student Book, Collins, AQA
- Potassium; iodide, page 115, GCSE Chemistry; Student Book, Collins, AQA
- Potassium; oxide, page 35, GCSE Chemistry; Student Book, Collins, AQA
- Potassium; sulphide, page 91, GCSE Chemistry; Student Book, Collins, AQA
- Potassium-40, page 122, GCSE Physics; Student Book, Collins, AQA