Difference between revisions of "Magnesium"
| (16 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
[[Magnesium]] is a [[metal]]. | [[Magnesium]] is a [[metal]]. | ||
==Key Stage 3== | ==Key Stage 3== | ||
| + | [[File:MagnesiumSymbol1.png|right|300px|thumb|The [[Chemical Symbol|chemical symbol]] for [[Magnesium]].]] | ||
| + | [[File:Mg-24_WK.PNG|right|200px|thumb|A 2 dimensional representation of a [[Magnesium]] [[atom]] with 12 [[proton]]s and 12 [[neutron]]s in the [[Atomic Nucleus|nucleus]] and 12 [[electron]]s orbiting the [[Atomic Nucleus|nucleus]].]] | ||
===Meaning=== | ===Meaning=== | ||
| − | |||
[[Magnesium]] is a [[Group 2]] [[element]], on the [[Periodic Table]], with an [[Atomic Number|atomic number]] of 12. | [[Magnesium]] is a [[Group 2]] [[element]], on the [[Periodic Table]], with an [[Atomic Number|atomic number]] of 12. | ||
===About Magnesium=== | ===About Magnesium=== | ||
| − | : [[Magnesium]] has the [[Chemical | + | ====Molecular Structure==== |
| + | : [[Magnesium]] has the [[Chemical Symbol|chemical symbol]] [[Magnesium|Mg]]. | ||
| + | : [[Magnesium]] [[atom]]s join together in large numbers to form a giant [[metal]] [[molecule]]. | ||
| + | ====Atomic Structure==== | ||
: [[Magnesium]] as 12 [[proton]]s and 12 [[neutron]]s in its [[Atomic Nucleus|nucleus]] giving it an [[Atomic Number]] of 12 and an [[Relative Atomic Mass|atomic mass]] of 24. | : [[Magnesium]] as 12 [[proton]]s and 12 [[neutron]]s in its [[Atomic Nucleus|nucleus]] giving it an [[Atomic Number]] of 12 and an [[Relative Atomic Mass|atomic mass]] of 24. | ||
| − | : [[Magnesium]] is | + | : An [[atom]] of [[Magnesium]] has only 2 [[electron]]s in its [[Outer Shell|outer shell]]. |
| + | ====Properties==== | ||
| + | : [[Magnesium]] is a more [[Reactivity|reactive]] [[Alkali Earth Metal|alkali earth metal]] than [[Beryllium]] but less [[Reactivity|reactive]] than [[Calcium]]. | ||
: [[Magnesium]] is more [[Reactivity|reactive]] than [[Carbon]] on the [[Reactivity Series|reactivity series]] so it must be [[Extraction of Metals|extracted]] from its [[ore]] using [[electrolysis]]. | : [[Magnesium]] is more [[Reactivity|reactive]] than [[Carbon]] on the [[Reactivity Series|reactivity series]] so it must be [[Extraction of Metals|extracted]] from its [[ore]] using [[electrolysis]]. | ||
| − | : [[Magnesium]] [[Chemical Reaction|reacts]] strongly with [[steam]] to produce [[Hydrogen]] [[gas]] and [[Magnesium Hydroxide]]. | + | : [[Magnesium]] [[Chemical Reaction|reacts]] slowly with [[liquid]] [[water]] and strongly with [[steam]] to produce [[Hydrogen]] [[gas]] and [[Magnesium Hydroxide]] and strongly with [[acid]] to produce a [[Magnesium]] [[salt]]s. |
| + | : [[Magnesium]] burns at a very high [[temperature]] with a bright white flame. | ||
: [[Magnesium]] is a [[solid]] at [[STP|room temperature]]. | : [[Magnesium]] is a [[solid]] at [[STP|room temperature]]. | ||
| − | + | ||
| − | |||
==Key Stage 4== | ==Key Stage 4== | ||
| + | [[File:MgKS4.PNG|right|200px|thumb|The [[Chemical Symbol|chemical symbol]] for [[Magnesium]].]] | ||
| + | [[File:Mg-24_WK.PNG|right|200px|thumb|A 2 dimensional representation of the [[Bohr Model]] of a [[Magnesium]]-24 [[isotope]] with 12 [[proton]]s and 12 [[neutron]]s in the [[Atomic Nucleus|nucleus]] and 2 [[electron]]s in the first [[Electron Orbital|shell]], 8 in the second and 2 in the [[Outer Shell|outer shell]].]] | ||
===Meaning=== | ===Meaning=== | ||
[[Magnesium]] is a [[Group 2]] [[element]], on the [[Periodic Table]], with 12 [[proton]]s in the [[Atomic Nucleus|nucleus]]. | [[Magnesium]] is a [[Group 2]] [[element]], on the [[Periodic Table]], with 12 [[proton]]s in the [[Atomic Nucleus|nucleus]]. | ||
===About Magnesium=== | ===About Magnesium=== | ||
| + | ====Molecular Structure==== | ||
: [[Magnesium]] has the [[Chemical Formula|chemical formula]] [[Magnesium|Mg]]. | : [[Magnesium]] has the [[Chemical Formula|chemical formula]] [[Magnesium|Mg]]. | ||
| + | : [[Magnesium]] [[atom]]s join together in a [[Giant Metallic Structure|giant metallic structure]]. | ||
| + | ====Atomic Structure==== | ||
: The most [[Stable Isotope|stable isotope]] of [[Magnesium]] has 12 [[neutron]]s in its [[Atomic Nucleus|nucleus]] giving it an [[Relative Atomic Mass|atomic mass]] of 24. | : The most [[Stable Isotope|stable isotope]] of [[Magnesium]] has 12 [[neutron]]s in its [[Atomic Nucleus|nucleus]] giving it an [[Relative Atomic Mass|atomic mass]] of 24. | ||
| − | |||
| − | |||
| − | |||
| − | |||
: An [[atom]] of [[Magnesium]] has only 2 [[electron]]s in its [[Outer Shell|outer shell]]. | : An [[atom]] of [[Magnesium]] has only 2 [[electron]]s in its [[Outer Shell|outer shell]]. | ||
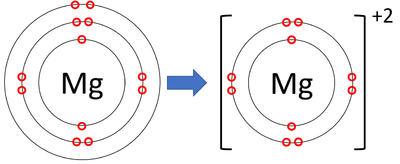
| − | : [[Magnesium]] [[ion]]s have lost two [[electron]]s to become [[Positive | + | : [[Magnesium]] [[ion]]s have lost two [[electron]]s to become [[Positive Charge|positively charged]]. |
| + | {| class="wikitable" | ||
| + | |- | ||
| + | |[[File:MagnesiumIonFormation.png|center|400px]] | ||
| + | |- | ||
| + | | style="height:20px; width:200px; text-align:center;" |A [[diagram]] showing the formation of a [[Magnesium]] [[ion]]. | ||
| + | |} | ||
| + | ====Properties==== | ||
| + | : [[Magnesium]] is a more [[Reactivity|reactive]] [[Alkali Earth Metal|alkali earth metal]] than [[Beryllium]] but less [[Reactivity|reactive]] than [[Calcium]].: [[Magnesium]] is more [[Reactivity|reactive]] than [[Carbon]] on the [[Reactivity Series|reactivity series]] so it must be [[Extraction of Metals|extracted]] from its [[ore]] using [[electrolysis]]. | ||
| + | : [[Magnesium]] [[Chemical Reaction|reacts]] slowly with [[liquid]] [[water]] and strongly with [[steam]] to produce [[Hydrogen]] [[gas]] and [[Magnesium Hydroxide]] and strongly with [[acid]] to produce a [[Magnesium]] [[salt]]s. | ||
| + | : [[Magnesium]] burns at a very high [[temperature]] with a bright white flame. | ||
| + | : [[Magnesium]] is a [[solid]] at [[STP|standard temperature and pressure]]. | ||
| + | |||
| + | ===References=== | ||
| + | ====AQA==== | ||
| + | |||
| + | :[https://www.amazon.co.uk/gp/product/0198359373/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0198359373&linkCode=as2&tag=nrjc-21&linkId=952a73bbb09d222ecc4b50d200679849 ''Magnesium, page 127, GCSE Biology; Third Edition, Oxford University Press, AQA ''] | ||
| + | :[https://www.amazon.co.uk/gp/product/0008158770/ref=as_li_tl?ie=UTF8&camp=1634&creative=6738&creativeASIN=0008158770&linkCode=as2&tag=nrjc-21&linkId=ec31595e720e1529e49876c3866fff6e ''Magnesium, page 287, GCSE Physics; Student Book, Collins, AQA ''] | ||
Latest revision as of 13:59, 19 February 2021
Contents
Key Stage 2
Meaning
Key Stage 3
Meaning
Magnesium is a Group 2 element, on the Periodic Table, with an atomic number of 12.
About Magnesium
Molecular Structure
- Magnesium has the chemical symbol Mg.
- Magnesium atoms join together in large numbers to form a giant metal molecule.
Atomic Structure
- Magnesium as 12 protons and 12 neutrons in its nucleus giving it an Atomic Number of 12 and an atomic mass of 24.
- An atom of Magnesium has only 2 electrons in its outer shell.
Properties
- Magnesium is a more reactive alkali earth metal than Beryllium but less reactive than Calcium.
- Magnesium is more reactive than Carbon on the reactivity series so it must be extracted from its ore using electrolysis.
- Magnesium reacts slowly with liquid water and strongly with steam to produce Hydrogen gas and Magnesium Hydroxide and strongly with acid to produce a Magnesium salts.
- Magnesium burns at a very high temperature with a bright white flame.
- Magnesium is a solid at room temperature.
Key Stage 4
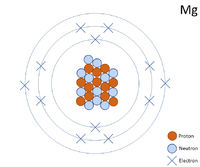
A 2 dimensional representation of the Bohr Model of a Magnesium-24 isotope with 12 protons and 12 neutrons in the nucleus and 2 electrons in the first shell, 8 in the second and 2 in the outer shell.
Meaning
Magnesium is a Group 2 element, on the Periodic Table, with 12 protons in the nucleus.
About Magnesium
Molecular Structure
- Magnesium has the chemical formula Mg.
- Magnesium atoms join together in a giant metallic structure.
Atomic Structure
- The most stable isotope of Magnesium has 12 neutrons in its nucleus giving it an atomic mass of 24.
- An atom of Magnesium has only 2 electrons in its outer shell.
- Magnesium ions have lost two electrons to become positively charged.
| A diagram showing the formation of a Magnesium ion. |
Properties
- Magnesium is a more reactive alkali earth metal than Beryllium but less reactive than Calcium.: Magnesium is more reactive than Carbon on the reactivity series so it must be extracted from its ore using electrolysis.
- Magnesium reacts slowly with liquid water and strongly with steam to produce Hydrogen gas and Magnesium Hydroxide and strongly with acid to produce a Magnesium salts.
- Magnesium burns at a very high temperature with a bright white flame.
- Magnesium is a solid at standard temperature and pressure.