Difference between revisions of "Ionic Compound"
| Line 38: | Line 38: | ||
| style="height:20px; width:200px; text-align:center;" |[[Nitrogen]] forms -3 [[ion]]s. | | style="height:20px; width:200px; text-align:center;" |[[Nitrogen]] forms -3 [[ion]]s. | ||
|} | |} | ||
| + | |||
| + | ===Bulk Properties=== | ||
| + | : In their [[solid]] [[State of Matter|state]] '''ionic compounds''' are poor [[Electrical Conductor|electrical conductors]] because the [[ion]]s are not free to move. | ||
| + | : In their [[liquid]] [[State of Matter|state]] '''ionic compounds''' are good [[Electrical Conductor|electrical conductors]] because the [[Charge|electrically charged]] [[ion]]s are free to move. | ||
| + | : Most '''ionic compounds''' are [[soluble]] in [[water]]. | ||
| + | : When [[dissolve]]d in [[solution]] '''ionic compounds''' are good [[Electrical Conductor|electrical conductors]] because the [[Charge|electrically charged]] [[ion]]s are free to move. | ||
| + | : '''Ionic compounds''' form [[Giant Ionic Structure|giant ionic structures]] which have high [[Melting Point|melting points]] due to the strong [[Electrostatic Force|electrostatic force]] between the [[ion]]s. | ||
Revision as of 14:23, 28 December 2018
Key Stage 4
Meaning
An ionic compound is a molecule formed from 2 or more elements which have transferred electrons to become ions.
About Ionic Compounds
- Ionic compounds form when atoms lose one or more electrons to become a positive ions and other atoms gain electrons to become negative ions. The electrostatic force of attraction between these ions is a strong chemical bond.
- Metal elements form positive ions because it is easier for them lose electrons than gain electrons to get a full outer shell. Metals are on the left hand side of the Periodic Table and usually have either 1, 2, 3 or 4 electrons in the Outer Shell.
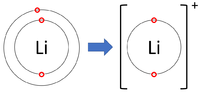
- Group 1 Elements all form +1 ions; Li+1, Na+1, K+1
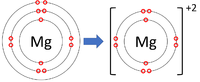
- Group 2 Elements all form +2 ions; Be+2, Mg+2, Ca+2
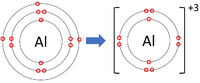
- Group 3 Elements all form +3 ions; Al+3
- Transition Metal Elements can form different ions which are shown by Roman Numerals; Iron can form Fe(II) which is Fe+2 or Fe(III) is Fe+3,
Manganese can form Mn(II) which is Mn+2 or Mn(IV) which is Mn+4
- Non-metal elements form negative ions because it is easier for them to gain electrons than lose electrons to get a full outer shell. Non-metals are on the right hand side of the Periodic Table and usually have 4, 5, 6, 7 or 8 electrons in their outer shell.
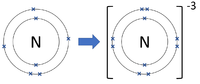
- Group 5 Elements all form -3 ions; N-3, P-3
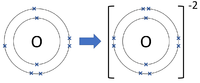
- Group 6 Elements all form -2 ions; O-2, S-2
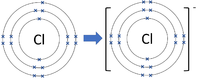
- Group 7 Elements all form -1 ions; F-1, Cl-1
- Some covalent compounds can form negative ions; Carbonate forms -2 ions CO3-2, Sulphate forms -2 ions SO4-2, Nitrate forms -1 ions NO3-1
Examples
| Lithium forms +1 ions. | Magnesium forms +2 ions. | Aluminium forms +3 ions. |
| Chlorine forms -1 ions. | Oxygen forms -2 ions. | Nitrogen forms -3 ions. |
Bulk Properties
- In their solid state ionic compounds are poor electrical conductors because the ions are not free to move.
- In their liquid state ionic compounds are good electrical conductors because the electrically charged ions are free to move.
- Most ionic compounds are soluble in water.
- When dissolved in solution ionic compounds are good electrical conductors because the electrically charged ions are free to move.
- Ionic compounds form giant ionic structures which have high melting points due to the strong electrostatic force between the ions.